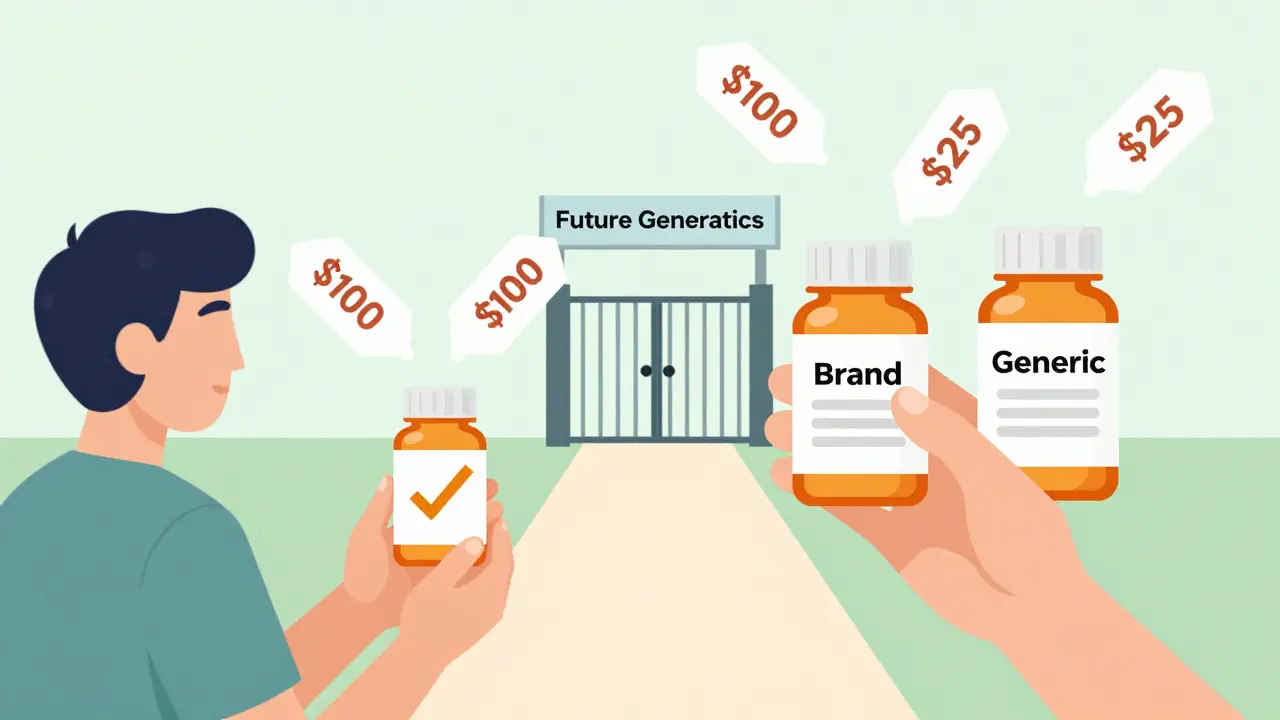
When a generic drug hits the market, it’s supposed to shake things up. Lower prices. More choices. Faster access. But in the U.S., there’s a hidden twist: the brand-name company can launch its own version of the generic - same pill, same factory, same active ingredient - just without the brand name. And it can do this while the first generic company still has its 180-day legal monopoly. This isn’t a glitch. It’s the system.
What Is the 180-Day Exclusivity Rule?
The 180-day exclusivity rule comes from the Hatch-Waxman Act of 1984. It was meant to reward the first generic company that challenges a brand-name drug’s patent. If they win in court or force a settlement, they get a half-year head start on selling their version. During that time, the FDA can’t approve any other generic versions of the same drug. That’s the incentive: risk millions in legal fees, win the patent fight, and get a lucrative monopoly.This isn’t theoretical. A single successful challenge can mean hundreds of millions in revenue. For a small generic manufacturer, it might be the difference between survival and shutdown. The rule was designed to make patent challenges worth the cost - which can run $2 million to $5 million per drug.
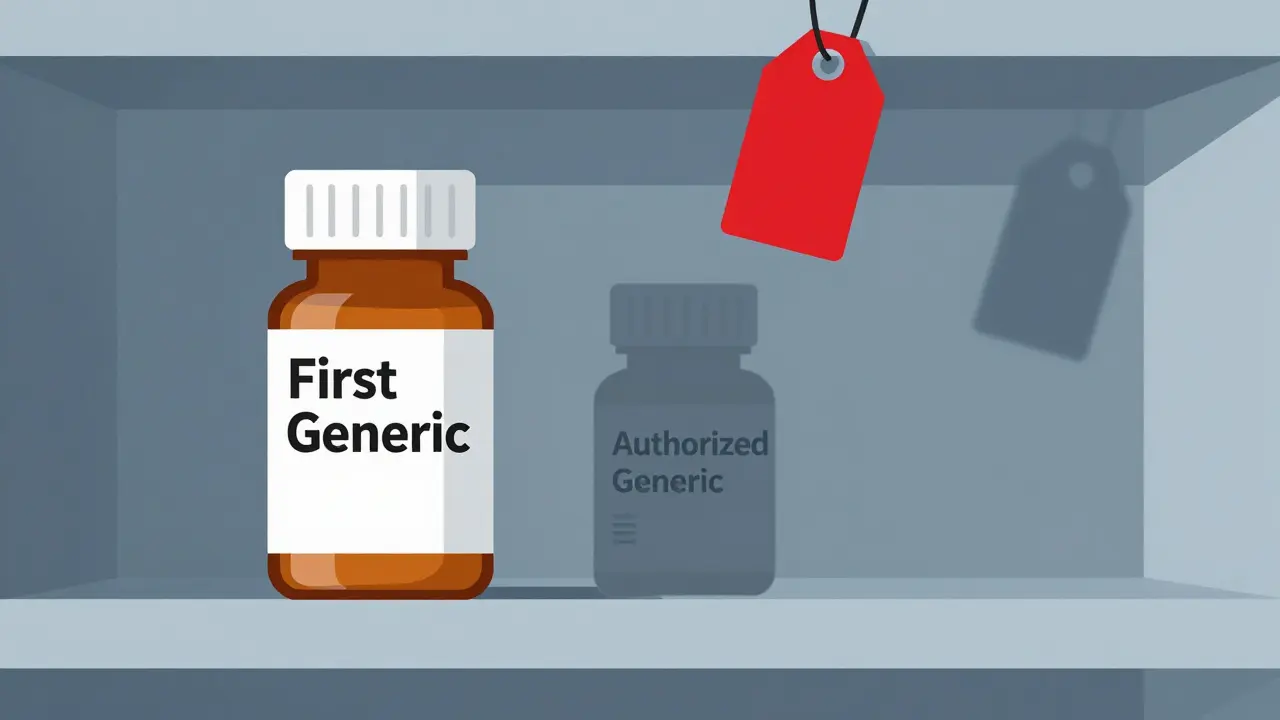
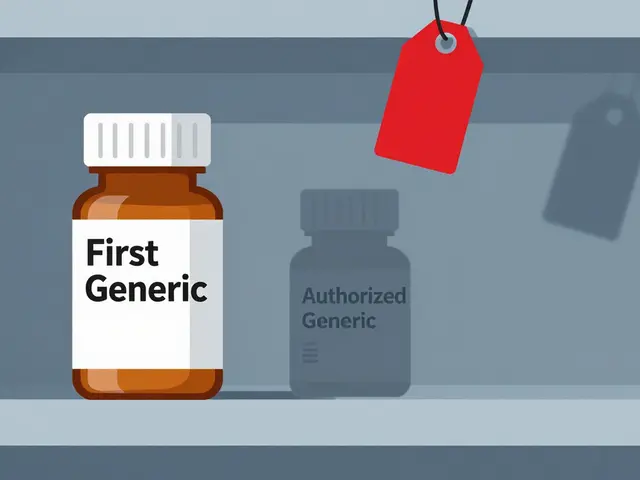
Authorized Generics: The Brand’s Secret Weapon
Here’s where things get messy. An authorized generic isn’t a copy. It’s the exact same drug made by the brand-name company, just sold under a different label. No new approval needed. No bioequivalence studies. Just repackage the brand drug and slap on a generic label. And they can launch it the same day the first generic hits shelves.That’s not just allowed - it’s legal. The FDA doesn’t block it. Courts have upheld it. And it happens more than you think. Between 2005 and 2015, brand-name companies launched authorized generics in about 60% of cases where 180-day exclusivity was granted. In 2019, Teva lost an estimated $287 million when Eli Lilly launched an authorized generic of Humalog during Teva’s exclusivity window.
For the first generic, the impact is brutal. Instead of capturing 80% of the market, their share often drops to 50% or lower. Revenue plummets by 30% to 50%. The exclusivity period, meant to be a reward, becomes a race against the very company it was designed to outmaneuver.
Why Does This Keep Happening?
The Hatch-Waxman Act never said brand-name companies couldn’t sell their own generic. It only protected the first generic applicant from other generics - not from the original maker. That loophole was never closed. And brand-name companies quickly realized the advantage.They don’t need to wait. They already have the drug made. They already have the distribution network. They can undercut the first generic on price, even while it’s still under exclusivity. And because they’re the original manufacturer, pharmacies and insurers often don’t even notice the switch. The pill is the same. The cost is lower. Why wouldn’t they?
Some argue this benefits patients. A 2021 RAND study found that when an authorized generic enters alongside the first generic, prices drop 15% to 25% more than if only one generic is on the market. That’s real savings for consumers. But for the generic company that spent years and millions to get there, it feels like a betrayal.

Legal Battles and Settlements
Most first generic applicants don’t just roll over. They fight back - often in the form of patent settlements. Drug Patent Watch found that 78% of first applicants now include clauses in their settlement agreements that block the brand from launching an authorized generic during the exclusivity window. These clauses aren’t always legal. The FTC has sued brand-name companies over “pay-for-delay” deals where the brand pays the generic to stay off the market. But authorized generic restrictions are trickier. They’re not direct payments - they’re contractual restrictions.Still, courts are starting to take notice. In 2022, the FTC filed its 15th antitrust lawsuit over authorized generics, arguing they’re a form of anti-competitive behavior. The agency believes these practices delay true competition and inflate prices long-term. The FDA’s Commissioner Robert Califf echoed this in 2023, saying the current system creates “unintended disincentives for timely generic entry.”
What’s Changing? The Push for Reform
There’s growing pressure to fix this. The Preserve Access to Affordable Generics and Biosimilars Act has been reintroduced in Congress multiple times since 2009. It would ban brand-name companies from launching authorized generics during the 180-day window. If passed, it could boost first generic revenue by 35%, according to the FTC. Industry analysts estimate that would make patent challenges worth an extra $150 million to $250 million per drug - potentially increasing the number of challenges by 20% to 25%.But the brand-name industry pushes back. PhRMA argues that blocking authorized generics would reduce competition and raise prices. They point to the same RAND study that shows lower prices when both versions are on the market. They say patients win - and they’re not wrong. But the system was never meant to let the brand compete directly with the challenger it was supposed to lose to.
What This Means for Generic Manufacturers
If you’re a generic company planning a Paragraph IV challenge, you’re not just preparing a drug application. You’re preparing for war. You need legal teams, regulatory experts, and commercial strategists working together months in advance. You need to know: Will the brand launch an authorized generic? Can you negotiate a ban? Can you afford to lose half your projected revenue?Many smaller companies now avoid these challenges entirely. The risk is too high. The costs are too steep. The reward is too uncertain. Between 2018 and 2022, 28% of first applicants lost part of their exclusivity due to procedural errors - like misjudging when the clock started. The FDA says the exclusivity clock begins when the drug is first shipped to customers - not when it’s approved. Miss that timing by a day, and you lose your advantage.
What This Means for Patients and Payers
Patients get lower prices faster - but sometimes not as fast as intended. The first generic might enter, but if an authorized generic follows immediately, the price drop is sharper. That’s good. But the long-term effect? Fewer generic companies are willing to challenge patents. That means fewer new generics overall. And fewer challengers means brand-name drugs stay protected longer. The system was supposed to break monopolies. Now, it sometimes reinforces them.Insurers and pharmacies see more options, but the competition is distorted. They’re not choosing between two independent companies. They’re choosing between two versions of the same product from the same manufacturer. That’s not true competition. It’s a controlled rollout.
The Bigger Picture
Since 1984, the Hatch-Waxman Act has saved the U.S. healthcare system over $2.2 trillion. That’s undeniable. But the 180-day exclusivity rule, once a powerful engine for generic entry, is now a system under strain. The original balance - innovation vs. access - is tipping. The incentives are misaligned. The law didn’t account for brand-name companies turning their own products into generics.The FDA approves both. The courts allow it. The market adapts. But the spirit of the law? It’s fading. And without legislative action, the next generation of generic drugs may never get the chance to challenge the giants. The first entrant won’t get their reward. The brand won’t lose its grip. And patients? They’ll still get cheaper drugs - but the path to get there is getting narrower.







Jody Patrick December 15, 2025
This is corporate theft dressed up as policy. The brand gets to keep its monopoly while pretending to help patients. No wonder generic companies are going under.
Radhika M December 16, 2025
Let me explain simple. First generic spends millions to fight patent. Then brand launches same drug under new label. First generic gets crushed. Patients save money, but fewer companies will risk it next time. System broken.
Raven C December 17, 2025
One must wonder-does the FDA’s acquiescence to this regulatory arbitrage not constitute a profound dereliction of fiduciary duty toward the public trust? The Hatch-Waxman Act, a monument to legislative compromise, has been eviscerated by corporate malfeasance masquerading as market efficiency.
Jigar shah December 17, 2025
This is a fascinating case of unintended consequences. The law intended to encourage competition, but the loophole allows the original monopolist to undercut the challenger. It’s like giving someone a head start in a race, then letting the other runner use their same shoes. The math doesn’t add up for small generics.
Marie Mee December 18, 2025
They're all in on this scam the big pharma and the FDA are in cahoots nobody talks about the real reason they let this happen is because they want us to stay sick so we keep buying drugs and the government gets kickbacks from the pharma companies and the doctors get paid to push the expensive ones
Naomi Lopez December 19, 2025
The irony is thick here. The system was designed to break monopolies, yet it’s now the primary tool for their reinforcement. It’s not innovation-it’s legal mimicry. And it’s not even clever, just lazy.
Victoria Rogers December 21, 2025
Actually the data shows prices drop more when authorized generics enter so stop crying about fairness. The market works. If you can't compete you shouldn't be in it. Also the FDA approves both so stop pretending this is shady. It's legal. Deal with it.
Jane Wei December 22, 2025
So basically the brand just slaps a new label on their own drug and calls it a generic? Wild. Feels like a magic trick where the audience is the patient and the wallet.
Nishant Desae December 23, 2025
Hey everyone, I just want to say that I really appreciate how this thread is bringing up such an important issue. I know it's complicated, and I know some of us feel frustrated, but I think if we keep talking about this with kindness and patience, maybe we can find a way to make sure the little guys aren't crushed by the big ones. I’ve worked with small generic manufacturers before, and they’re not just businesses-they’re people trying to do good. Let’s not forget that.
Philippa Skiadopoulou December 25, 2025
Authorized generics are a legitimate market mechanism under current regulatory interpretation. The Hatch-Waxman Act does not prohibit them. The issue is not legality but policy design. Legislative reform is required, not litigation.
Jonathan Morris December 26, 2025
This isn’t a loophole-it’s a backdoor cartel. The FDA, the courts, the big pharma lobby-they all work together to keep prices high. The 180-day exclusivity is a joke. They let the first generic in just long enough to make it look like competition, then crush them with their own version. It’s orchestrated. And you’re all being played.
Linda Caldwell December 27, 2025
Change is possible. We’ve seen it before. When people speak up, when lawmakers listen, when patients demand transparency-things shift. Don’t give up. The next reform could be the one that finally levels the field.
Anna Giakoumakatou December 28, 2025
Oh, so the brand gets to be both the referee and the player-and we’re supposed to cheer? How quaint. The only thing more ironic than this system is the fact that we call it ‘free market’ while the rules are written by the same people who own the game.
CAROL MUTISO December 29, 2025
You know what’s wild? This whole thing feels like watching someone steal your recipe, then sell it as their own ‘budget version’ while you’re still in the kitchen washing the pans. The original chef poured their soul into it-now they’re getting undercut by the same kitchen they trained in. And the customers? They just want a good meal at a fair price. But someone’s gotta pay the cost of innovation. Someone always does. And it’s never the ones who wrote the rules.