When a batch of medicine is released to patients, no one should be deciding whether it’s safe based on how fast the line was running or how much overtime the team worked. That’s the whole point of a quality assurance unit - it’s not there to help production meet targets. It’s there to stop production if something’s wrong. And that only works if it’s truly independent.
What Exactly Is a Quality Assurance Unit?
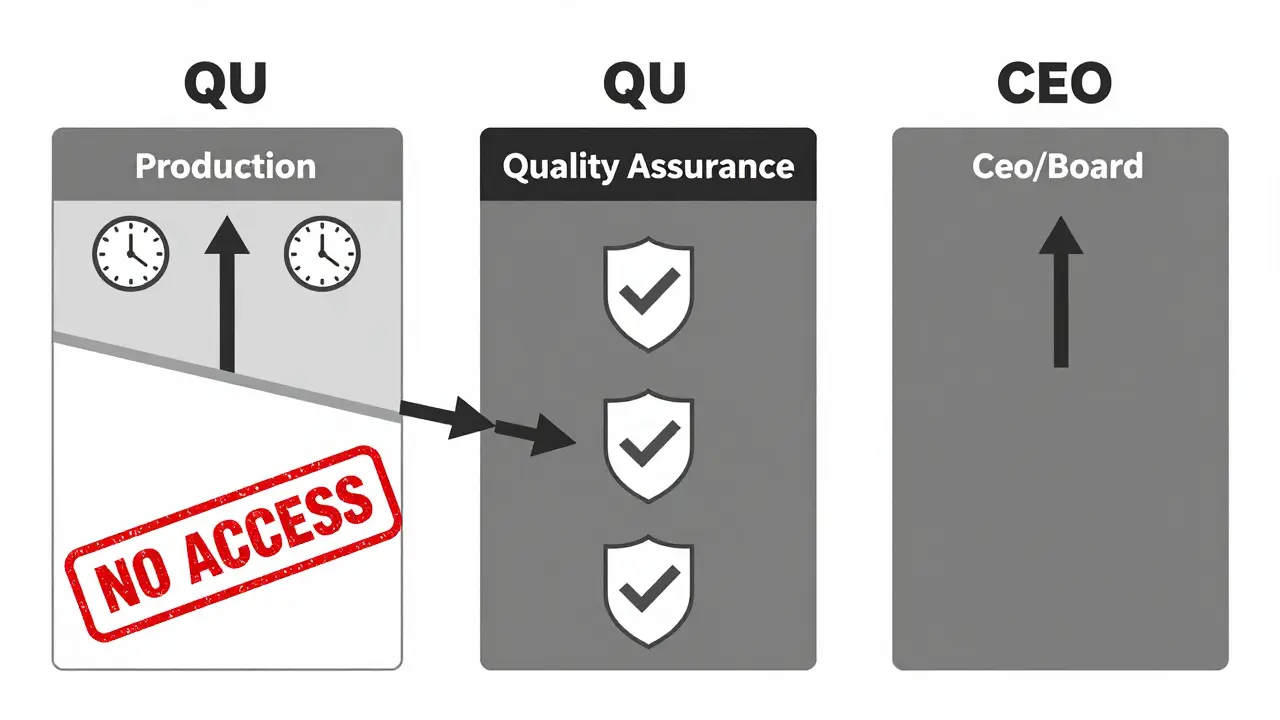
A Quality Assurance Unit (QU) is a formal, legally required team within a manufacturing facility that has the final say on whether a product can be released. It doesn’t run the machines. It doesn’t schedule shifts. It doesn’t report to the plant manager. Its only job is to protect quality - no exceptions. In pharmaceuticals, this isn’t optional. The U.S. Food and Drug Administration (FDA) requires it under 21 CFR 211.22. The QU must approve every component, every container, every label, and every finished batch before it leaves the facility. In nuclear plants, the same principle applies - independent oversight prevents catastrophic failures. This isn’t a best practice. It’s a legal requirement. The key word here is independent. That means the QU can’t be part of the production team. It can’t be managed by someone who’s under pressure to hit output targets. If the same person who’s responsible for making 10,000 units a day is also responsible for deciding if those units are safe, the system is broken before it even starts.Why Independence Isn’t Just a Good Idea - It’s a Legal Requirement
The FDA made this crystal clear in its 2006 guidance: “Quality decisions must remain objective and focused on product quality rather than production metrics.” Since then, warning letters have piled up. In 2024, 68% of all FDA warning letters issued to pharmaceutical manufacturers cited failures in QU independence. That’s up from just 29% in 2020. One common violation? Having the production manager also serve as the quality manager. A Reddit user in r/PharmaEngineering shared a real case: after their company merged the roles, two critical deviations slipped through - both were released without proper investigation. The batch went out. Patients were at risk. The company got fined. The QU was blamed. But the real problem? The structure. The same thing happens in nuclear facilities. After Three Mile Island, the International Atomic Energy Agency (IAEA) mandated independent oversight. Why? Because when safety is tied to output, corners get cut. And in these industries, corners kill.How Independence Actually Works in Practice
An independent QU doesn’t just review paperwork. It has real power:- It can reject a batch - even if the plant manager says it’s fine.
- It can halt production if a process drifts out of spec.
- It can demand root cause investigations, even if it delays shipments.
- It reports directly to the CEO or Board of Directors - not to the production head.
The Cost of Not Being Independent
When QU independence fails, the consequences aren’t abstract. They’re real, measurable, and costly. - Facilities with integrated quality and production teams have 63% more data integrity violations - the kind that lead to FDA warning letters and product recalls. - Organizations with truly independent QUs resolve critical quality issues 28% faster because they’re not stuck in approval loops with production managers. - Small facilities (under 50 employees) are more than twice as likely to fail QU independence checks. Why? They can’t afford full-time staff. But cutting corners here doesn’t save money - it risks lawsuits, recalls, and shutdowns. A 2024 FDA analysis found that facilities with a QU-to-production staff ratio below 1:15 had 3.2 times more repeat deviations. That’s not coincidence. It’s systemic failure. And then there’s the human cost. When a contaminated batch reaches patients, no one remembers the budget cuts or the efficiency goals. They remember the illness. The hospital stay. The loss of trust.How Small Companies Can Stay Compliant
You don’t need 50 people to have an independent QU. But you do need structure. Many small manufacturers in emerging markets struggle because they can’t hire a full-time quality team. The solution? Third-party oversight services. This is a growing market - growing at 14.2% annually - where external auditors provide independent review, batch release authority, and regulatory expertise. Some companies use a “quality ambassador” model. Eli Lilly trained production staff in quality principles but kept the final approval authority with a separate QU. Result? A 40% improvement in quality culture without sacrificing independence. The key is documentation. If your QU doesn’t have a clear org chart showing it reports to the CEO - not the plant manager - you’re already in violation. 95% of FDA warning letters cite poor documentation of QU authority. That’s avoidable.
What Skills Do QU Staff Need?
This isn’t a job for someone who just knows how to fill out forms. QU staff need:- Deep knowledge of GMP regulations - 100% of successful QU staff are trained in this.
- Statistical process control - 78% use it daily to spot trends before they become problems.
- Conflict resolution - 65% say their biggest challenge isn’t technical, it’s convincing production teams that saying “no” is part of their job.





Alec Stewart Stewart February 2, 2026
Just saw a batch get pulled last week because the QU said no - even though the plant was 2 days behind. No drama, no yelling. Just a quiet ‘this isn’t safe’ and the whole thing got fixed. That’s the kind of calm strength we need more of. 🙌
Susheel Sharma February 3, 2026
Let’s be brutally honest - most ‘independent’ QUs are paper tigers. They exist to tick boxes while the real power still lies with the production VP who has a bonus tied to output. The FDA doesn’t care about your org chart if your QU’s office is next to the machine shop. This whole system is theater.
Prajwal Manjunath Shanthappa February 4, 2026
One must ask: Is the very notion of ‘independence’ even tenable in a capitalist enterprise where shareholder value trumps all? The FDA’s regulations are laudable - but they are, at their core, a moralistic fantasy. A QU cannot be truly independent if it is funded, housed, and employed by the very entity it purports to oversee. This is not compliance - it is institutional hypocrisy.
Joseph Cooksey February 6, 2026
It’s funny how people act like this is some revolutionary idea - but if you’ve ever worked in pharma, you know this has been the law since the 80s. The real tragedy isn’t that companies ignore it - it’s that they’ve gotten so good at faking it. They create a ‘QU’ with one overworked person who signs off on everything because they’re scared to lose their job. Then they pat themselves on the back for being ‘compliant.’ You’re not independent if your paycheck comes from the guy you’re supposed to stop.
Justin Fauth February 6, 2026
USA built this system. We lead the world in safe meds. If you’re cutting corners overseas and calling it ‘cost efficiency,’ you’re not just breaking rules - you’re betraying patients. This isn’t a debate. It’s a duty. And if your country can’t handle it, then maybe your meds shouldn’t be sold here. Period.
Lorena Druetta February 7, 2026
Quality assurance is not a department. It is a covenant. A promise to every person who takes a pill that they will not be harmed by negligence. This is not a bureaucratic formality - it is a sacred obligation. We owe it to the sick, the elderly, the children. To compromise this is to betray humanity itself.
Daz Leonheart February 9, 2026
my first job in QA, the plant manager told me ‘if you stop the line again, you’re fired.’ i stopped it anyway. turned out the autoclave was reading 2 degrees off. saved 300k in recalls. never got fired. got promoted. sometimes you just gotta do the right thing, even if it’s scary.
Nathan King February 10, 2026
It is imperative to note that the structural integrity of quality assurance units is not merely a regulatory concern, but a foundational pillar of public health governance. The conflation of operational efficiency with safety assurance constitutes a profound epistemological failure - one that renders the entire quality control paradigm untenable. The IAEA and FDA frameworks are not suggestions; they are axiomatic imperatives.
rahulkumar maurya February 11, 2026
Small companies? Please. If you can’t afford a full-time QU, you shouldn’t be manufacturing. This isn’t a startup garage project - you’re making life-or-death products. Outsourcing to a third party? That’s just outsourcing responsibility. And when the FDA comes knocking, you’ll be the one holding the bag while your ‘consultant’ disappears into the ether.
Demetria Morris February 12, 2026
It’s disgusting how many people treat this like a cost center. People die because someone thought ‘we can squeeze one more batch out.’ If your CFO is involved in quality decisions, you’re not a company - you’re a death sentence waiting to happen.
Geri Rogers February 12, 2026
Y’all are talking about systems - but let’s talk about PEOPLE. The QU person who says NO? They’re the quiet hero who stays late, fights the boss, and gets zero credit. We need to celebrate them. Give them bonuses. Let them sit at the table. They’re not the enemy - they’re the shield. 💪❤️