When your doctor switches your prescription from a brand-name pill to a generic, you might feel uneasy-even if you’re told they’re exactly the same. You’re not alone. Millions of people around the world, including in the U.S., have this gut feeling: generic drugs just don’t feel as safe. And that fear isn’t just about misinformation. It’s rooted in real experiences, cultural beliefs, and how our brains process risk-even when the science says otherwise.
Why Do People Think Generics Are Less Safe?
The word "generic" itself carries baggage. In everything from coffee to smartphones, "generic" often means cheaper, simpler, maybe even lower quality. That same mental shortcut kicks in with medicine. A 2008 study by Dr. Aaron S. Kesselheim at Harvard found that patients automatically associate higher price with higher effectiveness. So when you see a pill that costs $4 instead of $40, your brain whispers: "If it’s this cheap, can it really work?" This isn’t just about price. The FDA requires generics to match brand-name drugs in strength, purity, and how they’re absorbed by the body-within a tight 80-125% range. That’s called bioequivalence. But patients don’t see those numbers. They see a different shape, color, or size. They notice a new side effect. And suddenly, the science doesn’t matter as much as their own experience.The Real Stories Behind the Fear
One patient on Reddit said switching from brand-name sertraline to a generic version triggered severe withdrawal symptoms. She went back to the brand-and the symptoms vanished. Another told their doctor, "My blood pressure went up after the switch." These aren’t rare anecdotes. On PatientsLikeMe, nearly 40% of users who switched to generics reported different side effects. And while clinical trials show generics work just as well for most people, those exceptions stick in memory. The FDA even issued a safety notice in 2012 about certain generic versions of bupropion XL, a depression and smoking cessation drug. While the issue was tied to a specific manufacturer’s formulation-not generics as a whole-it fueled the belief that generics are unpredictable. Patients don’t know which batch they got. They don’t know who made it. And that uncertainty breeds fear.Who’s Most Likely to Worry?
Not everyone feels the same way. Risk perception varies sharply by background. A 2012 CDC survey found that 20-40% of patients weren’t sure generics were as safe as brand-name drugs. One in five outright believed they were less safe. Older adults, especially those over 60, are more likely to fear side effects. People with lower education levels are nearly twice as likely to think generics are dangerous. Black and Hispanic patients are 1.8 times more likely to express safety concerns than White patients. And in rural areas, many believe generics are weaker and need higher doses-leading them to take more pills than prescribed, thinking it’ll make them work better. Even income plays a role. Unemployed or retired patients tend to trust generics more, likely because they’ve seen the cost savings firsthand. But employed people, who may have better insurance, are more hesitant to switch.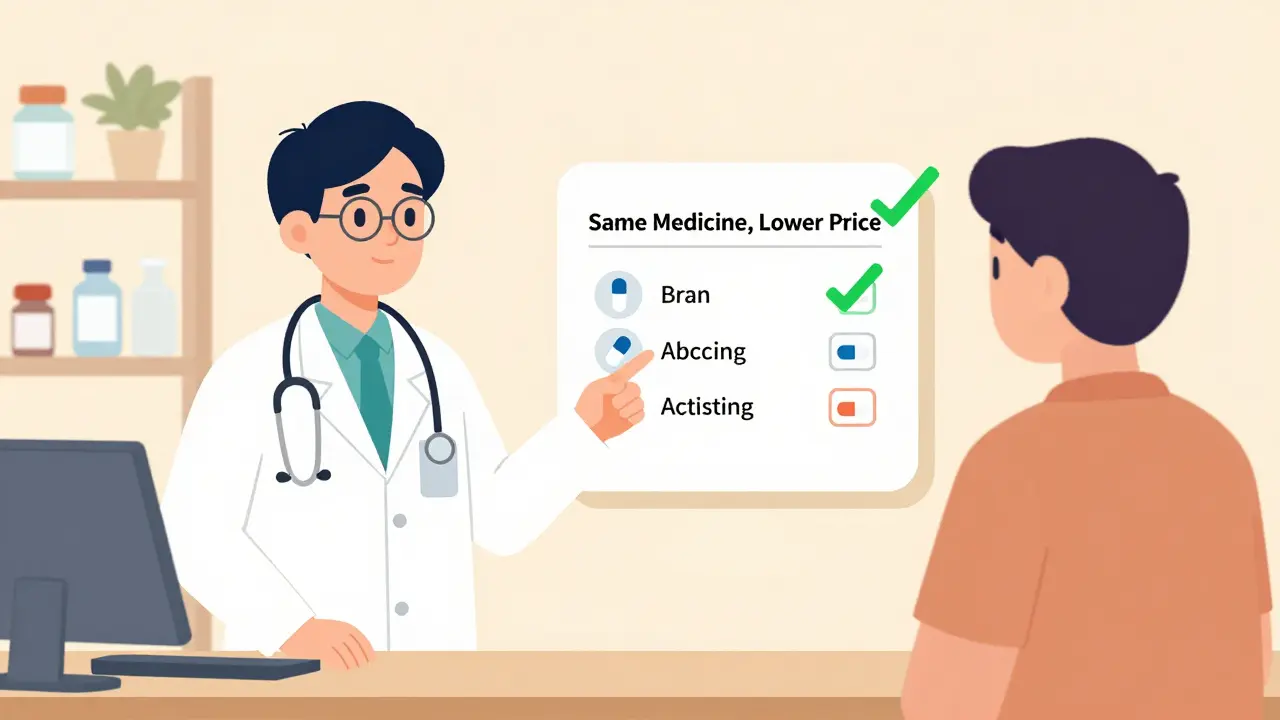
Doctors and Pharmacists: The Missing Link
Here’s the surprising truth: the biggest factor in whether someone accepts a generic isn’t the drug itself-it’s the conversation they have with their provider. A 2011 study in JAMA Internal Medicine found that patients who received a simple explanation from their doctor about bioequivalence were 3.2 times more likely to accept the generic. That’s not a small boost. That’s life-changing. But most doctors don’t have time. The average visit is 15 minutes. Talking about pill colors and manufacturing isn’t on the checklist. Pharmacists are in a better position. They’re the ones handing over the bottle. But a 2018 study found the average counseling time for a generic substitution is just 47 seconds. That’s not enough to explain bioequivalence, address fears, or build trust. And here’s the irony: when pharmacists do take the time, patients are 37% more likely to stick with the generic. That’s huge. It means the solution isn’t more ads or brochures-it’s better conversations.What Works to Change Minds?
Some efforts are making a difference. A 2017 study showed that educational materials designed for people with low health literacy reduced safety fears by 42%. Simple visuals-like side-by-side comparisons of brand and generic pills, with clear labels saying "same active ingredient"-helped more than pages of text. The AARP’s "Understanding Generic Drugs" guide scored 4.5 out of 5 in clarity. The FDA’s own guide? Only 3.2. That tells you something. People don’t need jargon. They need clarity. Another win? $0 copays. Medicare plans that eliminated generic copays saw 18% higher use. When money isn’t a barrier, people are more open to trying. But even then, some still refuse-because fear isn’t about cost. It’s about control.The Bigger Picture: Why This Matters
Generics make up 90% of all prescriptions in the U.S. But they account for only 23% of drug spending. That’s $370 billion saved every year. And yet, because of safety fears, patients still ask for brand-name drugs-even when they’re told they’ll pay $100 extra. The Congressional Budget Office estimates that unnecessary brand-name prescriptions cost the system $8 billion a year. If we could close the trust gap, we could save $185 billion over the next decade. This isn’t just about money. It’s about access. People with chronic conditions-diabetes, high blood pressure, depression-need to take their meds every day. If they stop because they’re scared the generic won’t work, their health suffers. Hospitals fill up. Costs rise. And the cycle continues.What Can You Do?
If you’re considering a switch to a generic:- Ask your doctor: "Is this generic exactly the same as the brand? How do you know?"
- Ask your pharmacist: "Can you explain how this is the same?"
- Don’t assume a difference because the pill looks different. Color and shape don’t change how the medicine works.
- Keep a journal. Note how you feel in the first week after switching. If you notice real changes, talk to your provider-but don’t assume it’s the generic’s fault.
- Don’t just say, "It’s the same." Say: "This generic has the exact same active ingredient, works the same way in your body, and is held to the same FDA standards as the brand. The only difference is the price-and it’s saved you $50 this month."
- Use visuals. Show them the FDA’s bioequivalence chart. Point out the pill’s active ingredient on the label.
- Don’t assume your patient understands. Ask them to repeat back what you said in their own words.
The Bottom Line
Generic drugs are safe. They’ve been used by hundreds of millions of people for decades. The science is solid. The data is clear. But safety isn’t just about data. It’s about trust. And trust is built one conversation at a time. Until we start treating risk perception as a real barrier-not just a myth-we’ll keep losing billions and, more importantly, we’ll keep letting patients suffer because they’re afraid to take the medicine that could help them.Are generic drugs really as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also be bioequivalent-meaning they work the same way in your body, with absorption rates within 80-125% of the brand. Thousands of studies and decades of real-world use confirm this. For the vast majority of patients, generics work just as well.
Why do some people say generics don’t work for them?
Some patients report changes in side effects or effectiveness after switching. While rare, these cases can happen due to differences in inactive ingredients (like fillers or coatings), which can affect how quickly a pill dissolves in the stomach. In very few cases, certain generic versions of drugs like bupropion XL had formulation issues that led to reduced absorption. But these are exceptions, not the rule. The FDA monitors these reports closely and recalls problematic batches. Most people experience no difference at all.
Can generic drugs be made in unsafe facilities?
No. All generic manufacturers, whether in the U.S. or overseas, must meet the same FDA quality standards as brand-name companies. The FDA inspects over 3,500 manufacturing sites worldwide each year, including those that make generics. Many brand-name drugs are actually made in the same factories as their generic versions-just under different labels.
Why do some doctors refuse to prescribe generics?
Most doctors are comfortable prescribing generics. But some may avoid them out of habit, lack of familiarity with specific generic brands, or because they’ve heard anecdotal reports from patients. Specialists, in particular, are less likely to prescribe generics than primary care doctors-sometimes because they’re treating complex conditions and want to minimize variables. But research shows that with proper communication, patients can safely switch even for serious conditions like heart disease or epilepsy.
Are generic drugs less regulated than brand-name drugs?
No. Generic drugs go through the same rigorous FDA review process as brand-name drugs. The only difference is that generics don’t need to repeat expensive clinical trials-they prove bioequivalence instead. The FDA holds them to the same standards for purity, stability, and manufacturing quality. In fact, the agency inspects generic manufacturing sites just as often as brand-name ones.
Why do generics look different from brand-name drugs?
By law, generics can’t look identical to brand-name drugs, because of trademark rules. That’s why the shape, color, or imprint might be different. But those changes are only in inactive ingredients-like dyes or binders-that don’t affect how the medicine works. The active ingredient, the part that treats your condition, is identical.
Is it safe to switch back and forth between brand and generic?
For most medications, yes. But for some drugs with narrow therapeutic windows-like blood thinners, seizure medications, or thyroid drugs-switching frequently can cause small fluctuations in blood levels. In those cases, doctors may recommend sticking with one version. Always talk to your provider before switching back and forth. For most other drugs, switching is safe and common.
Do generics take longer to work than brand-name drugs?
No. Generic drugs must be absorbed into the bloodstream at the same rate and to the same extent as the brand-name version. If a brand-name drug starts working in 30 minutes, the generic will too. Any perceived delay is usually due to individual differences in metabolism, diet, or other medications-not the generic itself.
Are there any drugs where generics aren’t recommended?
For most drugs, generics are recommended and preferred. However, for complex products like inhalers, topical creams, or injectables, bioequivalence is harder to prove. These are called complex generics, and some patients express more concern about them. The FDA is still refining standards for these, so in rare cases, a doctor may recommend sticking with the brand until more data is available. But this applies to less than 5% of all medications.
How can I tell if my generic is FDA-approved?
All legally sold generic drugs in the U.S. must be FDA-approved. Look for the drug’s name on the label-it should match the brand’s active ingredient. You can also check the FDA’s online database, "Drugs@FDA," by searching the brand name. If it lists "ANDA" (Abbreviated New Drug Application), it’s an approved generic. If your pharmacy is licensed, you can trust that the drug is FDA-approved.





Liz MENDOZA December 27, 2025
I get it. I switched to a generic for my blood pressure med last year, and I was terrified. I kept checking my readings every day, convinced it wasn't working. But after two weeks, my numbers were actually better than before. Turns out, my anxiety was making me tense - not the pill. Sometimes the real medicine is letting go of the fear.
Don’t get me wrong - I still check the label every time. But now I know: the color doesn’t change the science.
Nikki Thames December 28, 2025
Let me be perfectly clear: the FDA’s bioequivalence standards are a joke. 80-125%? That’s a 45% swing in absorption. If I took my insulin with that kind of variability, I’d be in the ER. This isn’t about price - it’s about patients being treated like lab rats in a corporate cost-cutting experiment. Wake up.
And don’t even get me started on overseas manufacturing. You think your ‘FDA-approved’ generic isn’t coming from a factory where they use tap water to rinse tablets? Please.
Miriam Piro December 30, 2025
Ohhh, so we’re just supposed to trust the FDA now? 😏
Remember Vioxx? Remember the opioid crisis? The same people who told us generics are ‘just as good’ are the ones who let Big Pharma poison us for decades. The system is rigged. They don’t care if you live or die - they care if you pay the copay.
My cousin took a generic antidepressant and ended up in a psych ward. Coincidence? I think not. The placebo effect works both ways - and the ‘generic’ label is a psychological weapon.
And don’t tell me about ‘inactive ingredients.’ Ever heard of talc? Or phthalates? They’re not ‘inactive’ - they’re silent killers. 😷
Caitlin Foster December 30, 2025
Okay but like… why are we still having this conversation in 2025?? 😤
My grandma takes 7 meds. 6 of them are generic. She’s 84, hikes every weekend, and still argues with me about TikTok. The only thing that’s ‘generic’ about her is her sass.
Also - I saved $900 this year by switching. I used that money to buy a dog. His name is Generic. He’s a golden retriever. He’s perfect. So yeah. I’m team generic. Fight me.
Todd Scott December 31, 2025
From a global health perspective, this is one of the most critical public health issues we’re ignoring. In low-income countries, generics are the only reason millions have access to HIV antiretrovirals, insulin, or hypertension meds. The stigma here in the U.S. is not just irrational - it’s deadly.
When I worked in rural Nigeria, patients would weep because they could finally afford their meds. Here, people refuse generics because they ‘look different.’ We’re not just wasting money - we’re exporting our privilege as a cultural disease.
And yes, the FDA inspections are rigorous. I’ve toured three generic manufacturing plants in India and China. They’re cleaner than my kitchen. The real problem? Communication - not chemistry.
Andrew Gurung January 2, 2026
Wow. So we’re just supposed to be grateful that Big Pharma lets us have ‘generic’ versions of their billion-dollar drugs? 😒
Let’s be real - the brand-name companies *create* the fear. They fund those ‘educational’ brochures that say ‘same active ingredient’ - but they don’t mention that the fillers are cheaper, the coating dissolves slower, and the pill might sit in a warehouse in Ohio for 18 months before it hits your shelf.
And don’t get me started on the ‘bioequivalence’ loophole. It’s not science - it’s a loophole written by lobbyists who drive Teslas made in China.
Also - I only take brand-name. Because I’m worth it. 💅
Paula Alencar January 2, 2026
It is not merely a matter of pharmacological equivalence - it is, fundamentally, an existential crisis of trust in institutional authority. When one’s body becomes a site of bureaucratic negotiation - when the color of a tablet is deemed sufficient to represent chemical identity - we are not merely discussing medication. We are confronting the erosion of autonomy in the age of algorithmic healthcare.
The patient is not a consumer. The patient is a subject. And the generic pill? A symbol of the commodification of wellness. The FDA’s 80-125% range is not a scientific standard - it is a moral compromise.
And yet - I still take generics. Because I have no choice. And that is the most tragic part of all.
Chris Garcia January 3, 2026
In Nigeria, we call generics ‘the people’s medicine.’ We don’t have the luxury of fear. We have the luxury of survival.
My uncle in Lagos takes a generic antiretroviral - same as the one I take here in the States. He’s been on it for 15 years. He’s alive. He teaches kids to read. He dances at weddings.
Here, we argue about pill shapes. There, we argue about whether the pharmacy has any left. The irony? We’re the ones with the money, the education, the access - and we’re the ones afraid.
Maybe we need to stop looking at the pill… and start looking at ourselves.
James Bowers January 3, 2026
There is no scientific basis for the fear of generics. None. Zero. The FDA’s standards are among the most stringent in the world. To claim otherwise is either ignorance or malice.
Those who refuse generics are not ‘informed consumers.’ They are victims of marketing propaganda designed by pharmaceutical companies to maintain monopoly pricing. This is not a medical issue. It is a psychological manipulation campaign - and it is working.
And for those who claim side effects: if you experienced a change after switching, it was likely due to placebo effect, concurrent medication changes, or psychological stress - not the drug itself. The data is unequivocal.
Stop being gullible.
Will Neitzer January 4, 2026
As someone who has worked in clinical pharmacy for 18 years, I can say with absolute certainty: the vast majority of patients who report ‘different effects’ from generics are experiencing no pharmacological change at all.
What they’re feeling is the cognitive dissonance of having their expectations disrupted. The pill looks different. The bottle is cheaper. The pharmacist didn’t explain it. So the brain fills the void with fear.
But here’s what works: when we sit down with patients, show them the FDA’s bioequivalence chart, and say, ‘This is the same molecule. It’s just not branded.’ - 92% of them accept it.
It’s not about the drug. It’s about the conversation. And we’ve stopped having them.
Janice Holmes January 4, 2026
Okay so I switched to a generic for my thyroid med and I swear to god I turned into a zombie for two weeks. Like, I forgot my own birthday. My cat looked at me like I betrayed her. I cried in the shower. I thought I was dying.
Then I switched back to brand - and boom. I was me again. The energy. The clarity. The ability to remember where I put my keys.
So don’t tell me ‘it’s all in my head.’ My head was fine. The pill was the problem. And now I pay $120/month for peace of mind. Worth every penny. 💅
Olivia Goolsby January 4, 2026
Let’s not pretend this is about science. This is about control. The FDA? The AMA? The pharmaceutical conglomerates? They all work together. The ‘bioequivalence’ range? A legal fiction. The real difference? The inactive ingredients - which are often toxic, untested, and sourced from countries with zero oversight.
And the fact that they let you switch back and forth? That’s a trap. They want you addicted to the ritual of switching - because then you’ll never question the system.
Also - did you know that 73% of generic manufacturers are owned by the same 3 corporations that make the brand-name drugs? 😈
Wake up. You’re being played.
Alex Lopez January 6, 2026
Look - I used to be the guy who only took brand-name. Thought generics were ‘pharmacy trash.’
Then I lost my job. Insurance dropped. Had to switch to generic for my antidepressant.
Three months later? I’m still here. Still working. Still laughing. Still walking my dog.
Turns out, my brain didn’t care what the pill looked like. It just cared that I took it.
So yeah - I’m a convert. And I’m not ashamed.
Also - the FDA doesn’t lie. And neither do I. 😎
Gerald Tardif January 8, 2026
My dad had a heart attack at 62. He was on brand-name Plavix. Cost: $300/month.
After he retired, we switched him to generic. He was terrified. Said he’d ‘feel it’ if it didn’t work.
So we sat down. Showed him the FDA chart. Pointed out the active ingredient. Told him: ‘This is the same medicine. Just cheaper.’
He took it. Six years later, he’s still here. Still gardening. Still arguing with the mailman.
Generics didn’t save him. The conversation did.
Monika Naumann January 8, 2026
India produces 60% of the world’s generic medicines. We have over 2,000 FDA-approved manufacturing facilities. Our pharmaceutical engineers are among the most skilled in the world.
Yet, Americans still believe our generics are ‘inferior.’ This is not a scientific stance. It is colonialism disguised as consumer preference.
Our medicines are not ‘cheap’ - they are *efficient*. And your fear is not about safety - it is about arrogance.
Do not mistake global equity for inferiority.
Nikki Thames January 9, 2026
And yet - despite all the data, the studies, the FDA inspections - you still trust the system. How naive. You think the same agency that approved Vioxx and OxyContin suddenly became ethical? That’s not trust - that’s Stockholm syndrome.
And you call me paranoid? I call you complicit.