It’s 2025, and you walk into your pharmacy to pick up your prescription. The pharmacist says, "The generic is FDA-approved, but we can’t get it yet." You’re not alone. Thousands of patients face this exact situation every month. The FDA says the drug is safe and ready. But it’s sitting on a shelf, locked behind a wall of patents. This isn’t a glitch. It’s the system working exactly as designed - and it’s costing patients billions.
Why FDA Approval Doesn’t Mean Availability
The FDA approved 63 first-time generic drugs by late September 2025. That sounds like progress. But here’s the catch: generic drug approval and generic drug availability are two completely different things. Between 2023 and 2025, the average time between FDA approval and actual market launch jumped to 3.2 years. That’s more than three years of patients paying full price for brand-name drugs while generics sit idle. This delay isn’t caused by slow manufacturing or supply chain issues - though those happen too. The real blocker is patent litigation. When a generic company files an application, it often certifies that the brand-name drug’s patents are invalid or won’t be infringed. That’s called a Paragraph IV certification. And when the brand company sues, the FDA is legally required to delay final approval for 30 months. That clock starts ticking the moment the lawsuit is filed - even if the patent is weak, outdated, or clearly meant to block competition.The Patent Thicket Strategy
Brand-name drugmakers don’t rely on one patent anymore. They build patent thickets - layers of overlapping protections around a single drug. In 2025, the average New Drug Application listed 14.7 patents. That’s up from 12.3 in 2020. Some drugs have over 50. Take Humira. Even though its core patent expired in 2016, AbbVie filed dozens of follow-up patents covering delivery devices, dosing methods, and manufacturing tweaks. These weren’t breakthrough innovations. They were legal shields. The result? A 10-year extension of market exclusivity. By 2025, Humira-related litigation involved 242 separate patents. This isn’t rare. A 2024 study in the Journal of Generic Medicines found that 68% of all generic applications in 2024 challenged at least one patent. And 72% of all generic launch delays were caused by litigation, not regulatory or manufacturing problems. The 30-month stay has become a strategic tool - not a safeguard. Companies file lawsuits even when they know they’ll lose, just to buy time.Who Gets Hit the Hardest?
Not all drugs face the same delays. Oncology drugs are the worst. The average wait between FDA approval and market access for cancer generics is 4.1 years. Compare that to cardiovascular drugs at 2.8 years and CNS medications at 2.3 years. Why? Because cancer drugs are expensive, complex, and profitable. Brand companies fight harder to protect them. Complex formulations - like injectables, inhalers, and transdermal patches - also get stuck longer. Eighty-nine percent of delayed complex generics face patent hurdles. Simple pills? Only 63% do. That’s because making a copy of a tablet is straightforward. Making a copy of a biologic inhaler? That’s a whole different science - and a bigger legal target. Small generic companies get crushed. Sixty-three percent of delayed generics come from firms with annual revenue under $500 million. They don’t have the legal teams or cash reserves to fight multi-year lawsuits. Big players like Teva and Sandoz can absorb the cost. Smaller ones? They fold. Or worse - they never start the fight at all.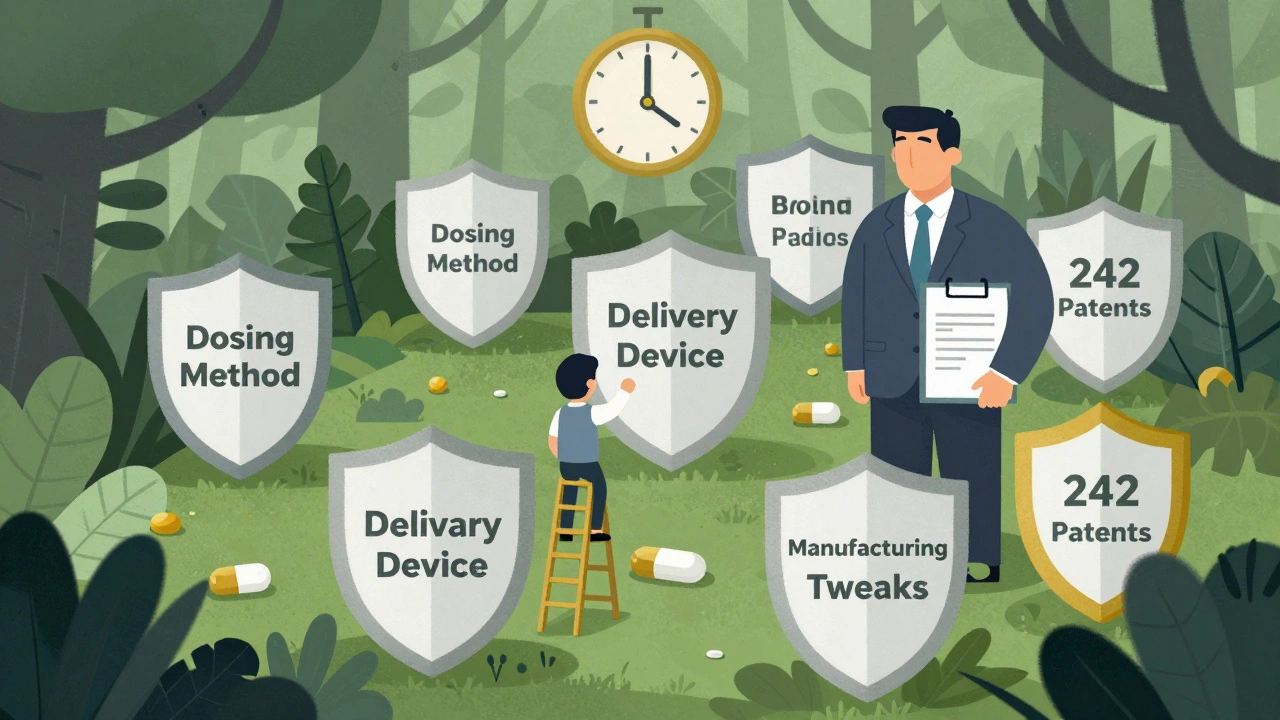
The Human Cost
Patients aren’t just inconvenienced. They’re forced to choose between their health and their finances. A 2025 survey by the Association for Accessible Medicines found that 82% of pharmacists get daily calls from patients asking why their approved generic isn’t available. The most common drugs? Eliquis, Trulicity, Steglatro, and Xarelto - all billion-dollar brands. Patients For Affordable Drugs Now documented 412 cases between 2023 and 2025 where people skipped doses or went without medication because the generic wasn’t on the shelf. The brand versions cost an average of $487 a month. The generics? Around $85. That’s a 500% price gap. Medicare Part D alone spent $3.2 billion extra in 2025 because of these delays. Hospital pharmacy directors reported that 78% of drug shortages in 2025 were worsened by patent delays - especially for oncology injectables. One pharmacist on Drugs.com wrote: "We had the generic for Xarelto approved last November. The brand company filed three new patents last month. Now we’re looking at another 30-month delay." That’s not an outlier. That’s the new normal.Why the FDA Can’t Fix This
The FDA can approve a drug. But it can’t force a patent holder to stop suing. That’s the law. The agency’s hands are tied by the Hatch-Waxman Act, passed in 1984. It was meant to balance innovation and access. Today, it’s being exploited. The FDA tried to help. In 2025, it launched an AI-assisted review system that cut approval times by 22% for non-litigated generics. It also pushed for cleaner Orange Book listings - the public database of patents linked to drugs. But patent listings are still messy. Brand companies list patents that don’t even cover the drug’s active ingredient. The FDA can’t remove them unless someone files a citizen petition - and those take 18.2 months on average to resolve. The new National Priority Voucher program speeds up reviews for certain drugs - but only if they’re not caught in litigation. Patent fights happen in court, not in the FDA’s lab. So the agency’s tools are useless against the real problem.
What’s Changing?
There are signs of pressure. The Federal Trade Commission filed seven enforcement actions between 2024 and 2025 against companies using patent thickets to block generics. One case against Jazz Pharmaceuticals over Xyrem ended in February 2025 with a settlement requiring earlier generic entry. The CREATES Act, which would make it easier for generic makers to get samples of brand drugs for testing, passed the House in 2024 but stalled in the Senate in 2025. Without it, companies can legally refuse to sell samples - another tactic to delay competition. The industry is shifting, too. The practice of brand companies launching their own "authorized generics" to undercut competitors has dropped from 28% in 2020 to just 12% in 2025. That’s a good sign - it means they’re relying more on legal tactics than market tactics. But the biggest change might come from the top. Dr. Peter Bach became FDA Commissioner in January 2025. He’s publicly called for greater transparency in patent listings and has signaled support for reforming the Orange Book. Analysts at William Blair predict that if these changes stick, generic entry could be cut by 8 to 12 months by 2027.What Can You Do?
If you’re a patient: Ask your pharmacist if your drug is approved but unavailable. If yes, ask why. Demand to know if the delay is patent-related. Share your story with patient advocacy groups. If you’re a prescriber: Know the difference between approval and availability. When a generic isn’t on the shelf, don’t assume it’s out of stock. It’s likely locked behind a lawsuit. Consider alternatives - or push back on insurers who force brand-name use. If you’re in the industry: Understand that patent litigation is now a cost of doing business. Leading generic companies spend $12.7 million per case on average. They’re diversifying suppliers - going from 1.8 API sources in 2022 to 3.4 in 2025 - to avoid supply chain delays on top of legal ones.What’s Next?
The $78.3 billion in annual brand sales from drugs losing exclusivity in 2025 is a massive target. The generic market hit $264.7 billion globally - but the U.S. is still missing out on billions because of delays. Without reform, the gap between innovation and access will keep widening. The Hatch-Waxman Act needs an update. Limiting the number of patents that can be listed per drug. Shortening the 30-month stay. Making patent listings more accurate. These aren’t radical ideas. They’re common sense. The system was built to help patients get affordable medicine. Right now, it’s helping drugmakers keep them out.Why is my generic drug approved but not available?
Even after the FDA approves a generic drug, it can’t be sold if the brand-name company files a patent lawsuit. This triggers a 30-month legal hold, called a statutory stay, that blocks the generic from launching - even if the patent is weak or outdated. This delay is not about safety or manufacturing. It’s a legal tactic used to extend market exclusivity.
What is a Paragraph IV certification?
A Paragraph IV certification is a legal statement made by a generic drug company when applying to the FDA. It says that the brand-name drug’s patents are either invalid or won’t be infringed by the generic version. This triggers a patent lawsuit from the brand company, which then starts the 30-month clock that delays the generic’s market entry.
How long do patent delays typically last?
The average delay between FDA approval and market launch for a generic drug is 3.2 years. For complex drugs like injectables or oncology treatments, delays can stretch to 4.1 years. The 30-month statutory stay is the main reason, but many cases involve multiple lawsuits that extend the timeline even further.
Which drugs are most affected by patent delays?
Oncology drugs, injectables, and high-revenue brand medications like Eliquis, Trulicity, Steglatro, and Xarelto face the longest delays. These are often drugs with high profit margins, so brand companies invest heavily in patent litigation to protect them. Complex formulations like inhalers and injectables are especially vulnerable because they’re harder to copy and easier to patent.
Is the FDA doing anything to fix this?
The FDA has improved its review speed for non-litigated generics using AI tools and is pushing for cleaner patent listings in the Orange Book. But it cannot stop patent lawsuits - those are handled by courts. The agency’s authority ends at approval. Until Congress changes the Hatch-Waxman Act, the FDA has limited power to speed up generic access when patents are in play.
Are biosimilars affected the same way?
Yes - even more so. Biosimilars face even more complex patent landscapes. For example, Humira’s patent battles involved 242 patents, delaying biosimilar entry for over a decade. The average number of patents challenged per biosimilar application rose from 5.2 in 2020 to 9.7 in 2025. While biosimilar approvals are increasing, litigation is slowing their market entry just like with small-molecule generics.
How much does this cost patients and Medicare?
The Congressional Budget Office estimated that patent-related delays cost Medicare Part D $3.2 billion in extra spending in 2025. Patients pay up to 500% more for brand-name drugs when generics are blocked. For drugs like Eliquis, that means paying $487 a month instead of $85. Many patients skip doses or go without treatment because they can’t afford the brand.







wendy b December 12, 2025
So let me get this straight-FDA says it’s safe, but we can’t sell it because some pharma execs are playing chess with patents? 😒 I mean, come on. This isn’t innovation. It’s extortion. And the worst part? Patients are the ones paying the price-in dollars, in health, in lives. Someone needs to burn this whole system down. #PharmaScam
Reshma Sinha December 13, 2025
From a global health equity lens, this is a textbook case of regulatory capture & IP rent-seeking. The 30-month stay has morphed from a procedural buffer into a strategic monopolization engine. We’re witnessing systemic failure in incentive alignment-where R&D rewards are being weaponized to suppress generic diffusion, especially in high-cost therapeutic classes like oncology. The CREATES Act isn’t enough; we need structural IP reform with sunset clauses on secondary patents. #HealthEquity #PatentReform
Lawrence Armstrong December 14, 2025
My cousin just had to skip her Eliquis doses last month because the generic was approved but locked up. 😔 She’s on Medicare and can’t afford $500/month. The FDA’s AI tools are cool, but they can’t fix a broken law. We need Congress to act-this isn’t rocket science. Just fix Hatch-Waxman before more people die. #GenericAccessNow
Donna Anderson December 15, 2025
OMG I didn’t realize this was happening so much!! I thought generics just took longer to make… but nooo, it’s lawsuits?? Like… why?? My mom’s on a generic for blood pressure and it took 2 years to show up. She cried when she finally got it. This is so messed up. We need to call our reps. Like, NOW.
Levi Cooper December 15, 2025
Let’s be honest-this is what happens when you let foreign companies and greedy startups undercut American innovation. The U.S. spends billions developing these drugs, and then some small firm from India files a Paragraph IV and suddenly we’re giving away the crown jewels? No. We protect our IP. If patients can’t afford it, they should’ve saved more. Or moved to India. Problem solved.
sandeep sanigarapu December 17, 2025
This system was designed for balance. Now it is weaponized. The 30-month stay was never meant to be a delay tactic. It is a legal loophole exploited by corporations. Simple solution: cap the number of patents per drug. Enforce real innovation. Protect patients. Not profits.
nikki yamashita December 17, 2025
Wait so the FDA approves it but we can’t sell it? That’s wild. I’m so glad I’m not the only one noticing this. My pharmacist says it’s getting worse every year. We need to make noise. Like, loud noise. 💪
Adam Everitt December 18, 2025
It’s not just about patents… it’s about the myth of meritocracy in pharma. We worship innovation, yet punish accessibility. The real tragedy? The patents aren’t even *good*. They’re legal smoke. We’ve confused intellectual property with moral right. And the cost? Human lives. Quietly, systematically, erased.
Rob Purvis December 18, 2025
Let’s not forget: the Hatch-Waxman Act was meant to be a compromise-innovation AND access. Now it’s just a shield for monopolies. The FDA can’t fix this. Only Congress can. And they won’t, unless we flood their offices with calls. Every. Single. Day. The numbers don’t lie: $3.2 billion wasted. 412 people skipping meds. 63% of small generics crushed. This isn’t policy. It’s predation. And it’s time to call it what it is.