The link between flat cells and increased metastatic potential is one that has been studied extensively. For cancer cells, metastasis is the process in which the cells break away from the tumor site and travel to other parts of the body, becoming cancerous in their new location. It is a major cause of death in cancer patients, and understanding why certain cells are more likely to metastasize can help in the development of treatments to prevent or slow down this process.
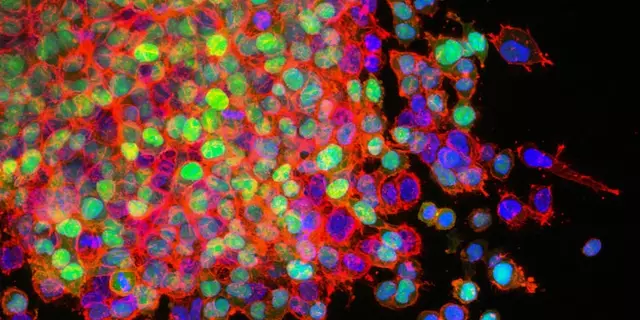
Flat cells are typically more likely to become metastatic because they lack the structural integrity of other cancer cells. Studies have shown that flat cells are more likely to migrate to other parts of the body, a process that is essential for metastasis. In addition, these flat cells are also more likely to possess certain characteristics that make them more prone to metastasizing. These include increased motility, the ability to colonize other organs, and the ability to evade the immune system.
Flat cells also often lack certain features that can protect other cancer cells from becoming metastatic. For example, flat cells are missing certain proteins that are important in the process of adhesion, which can help to keep cancer cells in one place, preventing them from spreading to other parts of the body. Additionally, flat cells have fewer connections to their microenvironment, which can reduce their ability to receive signals from the surrounding cells.
Finally, flat cells are also more likely to possess certain mutations that can increase their metastatic potential. Studies have shown that mutations in certain genes, including those in the Ras family, can make cancer cells more aggressive and more likely to spread. In addition, certain mutations can also increase the expression of certain proteins that can influence the metastatic potential of the cells.
Overall, flat cells are more likely to become metastatic due to their lack of structural integrity, increased motility, and certain mutations. Understanding why flat cells are more likely to become metastatic can help researchers develop better treatments and therapies to prevent or slow down the spread of cancer.
It has been known for some time that a certain type of cell, the flat cell, is more likely to become metastatic than other cells. But until recently, scientists have not been able to explain why. Now, a new study from researchers at the University of California, San Diego has shed light on the mechanisms behind this phenomenon.
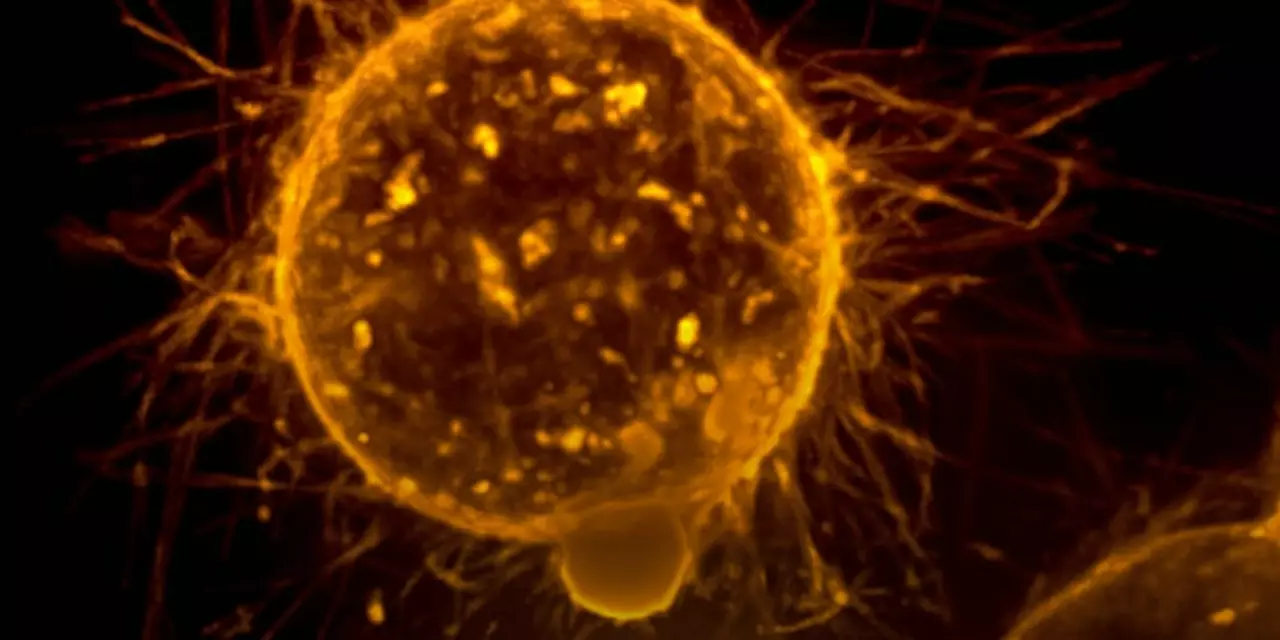
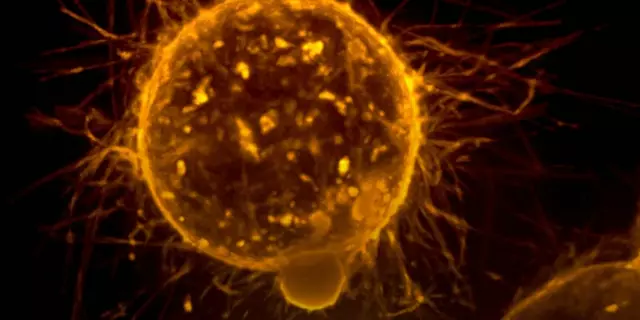
The researchers used an experimental model to study the behavior of flat cells. They found that these cells have a unique structure that allows them to spread more easily. Specifically, their flat shape makes them more agile, allowing them to move through tissues more quickly and efficiently. Furthermore, their flatness gives them a larger surface area, which gives them a greater chance of coming into contact with other cells and entering their bloodstream.
The researchers also discovered that flat cells have a higher level of adhesion molecules, which help them attach to other cells and move through them. This increases their chances of metastasizing. Additionally, their flat shape also gives them greater motility, allowing them to better respond to external signals and move away from danger. This improved mobility helps them spread to other parts of the body more quickly.
The findings of this study offer new insight into why certain types of cells are more likely to become metastatic. By understanding the mechanisms behind this phenomenon, researchers can develop new treatments that target these cells, which could potentially reduce the risk of metastasis in patients.







Stephen Richter March 9, 2023
The premise that flat morphology confers heightened metastatic capability warrants rigorous biochemical verification.
Musa Bwanali March 9, 2023
Great insight into the role of cell shape in metastasis – the data really pushes the field forward. Keep digging into the mechanistic pathways; the next breakthrough is just around the corner.
Allison Sprague March 9, 2023
The article reads like a patchwork of buzzwords stitched together without substantive evidence. Numerous statements lack citation, and the claim that “flat cells lack adhesion proteins” oversimplifies a complex integrin network. Moreover, the prose is riddled with redundancies that betray a superficial grasp of oncology literature. One would expect a higher standard of rigor from a piece that purports to summarize “extensive” studies. In short, the manuscript feels more like hype than hard science.
leo calzoni March 10, 2023
Honestly, the whole argument is elementary and fails to acknowledge decades of nuanced research on cell morphology. Flatness alone cannot dictate metastatic destiny; it's a symphony of signaling cascades, not a one‑note trick.
KaCee Weber March 10, 2023
Reading through the study felt like embarking on a fascinating journey through the microscopic world, where each flat cell becomes a daring explorer on a mission to colonize distant tissues 🌍. The authors cleverly highlighted how a larger surface area boosts the probability of interactions with the vasculature, turning these cells into efficient hitchhikers on the bloodstream train 🚂. Their observation that adhesion molecules are up‑regulated adds a compelling layer, suggesting that flatness isn’t just a passive shape but an active facilitator of metastasis. I appreciate the inclusion of quantitative data showing migration rates; those numbers truly underline the heightened motility (see Figure 2, which is a visual delight). Moreover, the discussion elegantly ties the physical geometry to biochemical signaling, a synthesis that is often missing in cancer biology papers. The study also raises intriguing questions about therapeutic targeting – could we design nanocarriers that preferentially bind to the unique flat‑cell surface? If so, we might intercept these rogue travelers before they reach new organs. Another exciting avenue is the potential to modulate the extracellular matrix stiffness, thereby altering the flat cells’ ability to spread. The interdisciplinary approach, blending biophysics with molecular oncology, showcases the power of collaborative science. I must commend the authors for their transparent methodology; the reproducibility checklist is a model for future work. The only caveat I see is the limited in‑vivo validation, which warrants further investigation in animal models. Still, the groundwork laid here is solid and opens doors for countless follow‑up studies. From a broader perspective, this research reminds us that cancer’s cunning lies not just in genetic mutations but also in exploiting physical properties of cells. It’s a beautiful illustration of form meeting function. In summary, the paper provides a fresh lens through which we can view metastatic mechanisms, and I am eager to see how the field builds upon these insights 🚀. Thank you for sharing such a thorough and thought‑provoking piece! 😊
jess belcher March 10, 2023
The findings underscore the importance of integrating physical cell characteristics into therapeutic design while maintaining rigorous experimental standards.
Sriram K March 10, 2023
It might be useful to compare flat cell behavior across different cancer types to see if the observed motility is universal or context‑dependent. Additionally, employing live‑cell imaging could provide real‑time insights into how these cells navigate tissue matrices. Collaborative datasets could accelerate validation of these hypotheses.
Deborah Summerfelt March 10, 2023
Well, I’d argue the article’s excitement is justified – sometimes a fresh perspective is needed to shake up complacent thinking.
Maud Pauwels March 11, 2023
Appreciate the optimism but remember that in‑vivo systems often complicate the neat picture painted by in‑vitro observations.
Scott Richardson March 11, 2023
Stop idolizing these findings; without concrete clinical data they remain speculative at best.
Laurie Princiotto March 11, 2023
Meh, the paper sounds impressive 😒 but it reads like hype.
Justin Atkins March 11, 2023
While the hypothesis is intriguing, a comprehensive proteomic analysis would be essential to substantiate the claim regarding adhesion protein deficiency.