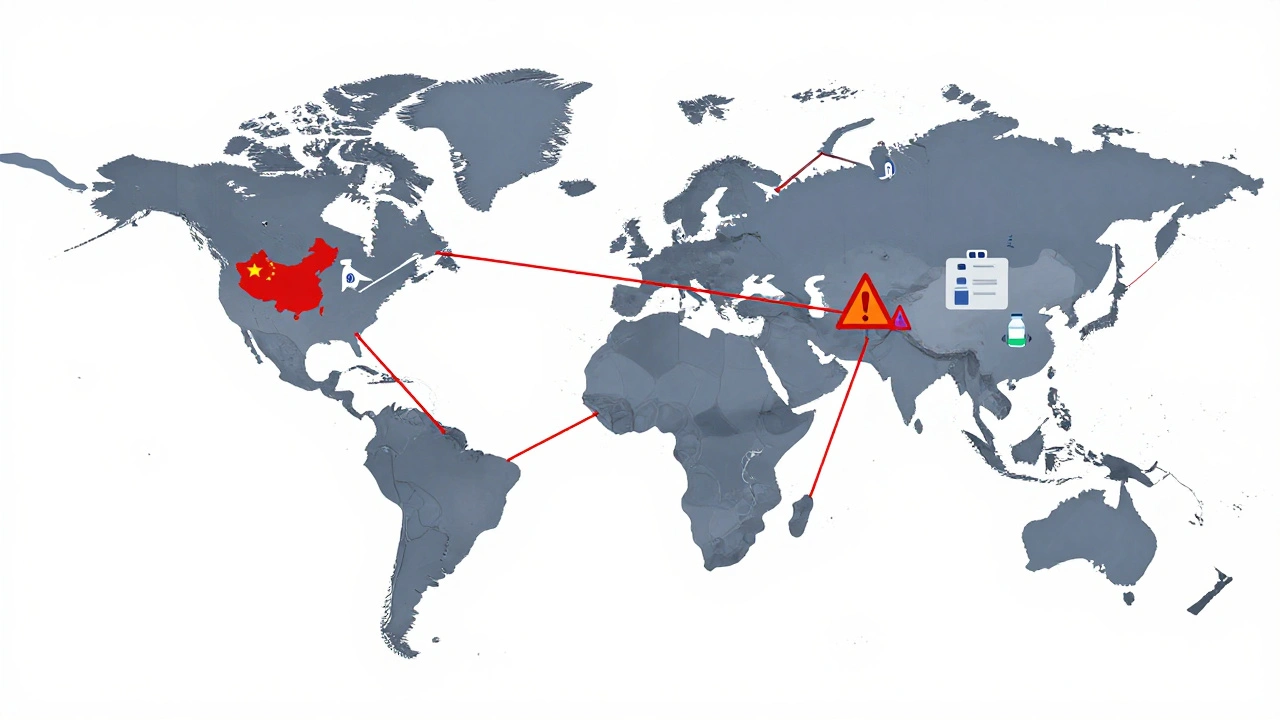
By 2025, if you’ve had trouble filling a prescription for antibiotics, blood pressure meds, or even basic painkillers, you’re not alone. It’s not because pharmacies are running low-it’s because the global system that makes those drugs has become dangerously fragile. More than 80% of the active ingredients in U.S. prescription drugs come from just two countries: China and India. And when something breaks in those supply chains-whether it’s a port strike, a political clash, or a factory shutdown-hospitals and clinics feel it first. The U.S. isn’t alone. Australia, Canada, and the EU are seeing the same pattern. This isn’t a temporary glitch. It’s the new reality of how medicine is made.
How Did We Get Here?
In the 1990s and early 2000s, drugmakers made a simple calculation: why make medicine at home when you can outsource it for half the cost? Raw materials like APIs (active pharmaceutical ingredients) were cheaper to produce in China. Packaging and final assembly? Easier and cheaper in India. Factories scaled up. Regulations loosened. Companies stopped asking where their ingredients came from-they just asked for the lowest price.
By 2025, that model has hit its limits. The average lead time for a shipment of active ingredients from China to the U.S. has jumped 50% since 2019. A container that used to take 18 days to cross the Pacific now takes 27. That’s not just a delay-it’s a domino effect. When a single API supplier in Shanghai shuts down for a week due to inspections, it can trigger shortages of dozens of medications across continents.
And it’s not just about distance. Tariffs added in 2024 hit $340 billion worth of imported pharmaceutical inputs. Some companies passed those costs to consumers. Others cut corners. The result? A 15% increase in quality-related recalls for foreign-made ingredients in 2024, according to the FDA. That’s not a glitch-it’s systemic.
The Drug Shortage Crisis
In 2024, the U.S. saw more than 300 drug shortages. That’s up from 180 in 2020. The most affected? Generic drugs. Why? Because they’re low-margin products. Companies don’t invest in backup suppliers for pills that earn them $0.05 per tablet. So when a Chinese factory gets hit by flooding-or when India bans exports of a key ingredient to protect its own supply-the pipeline breaks.
Take amoxicillin. It’s one of the most common antibiotics. Over 90% of its active ingredient comes from China. In early 2024, a fire at a supplier in Zhejiang province caused a nationwide shortage in the U.S. and Australia. Hospitals had to ration it. Pediatricians switched to more expensive alternatives. Parents waited days for refills. This wasn’t an accident. It was predictable.
Even life-saving drugs aren’t safe. Insulin, heparin, and cancer drugs all rely on foreign-sourced components. In 2025, a single supplier in India accounted for 70% of the raw material for a widely used chemotherapy agent. When regulatory inspectors flagged quality issues, the entire global supply chain slowed down. Patients missed treatments. Clinics delayed care. The human cost isn’t measured in dollars-it’s measured in missed doses and delayed diagnoses.
Why Reshoring Isn’t Easy
You might think the solution is simple: bring drug manufacturing back home. But it’s not that easy. Making pharmaceuticals requires specialized facilities, highly trained workers, and strict regulatory oversight. Building a single FDA-compliant API plant in the U.S. costs between $200 million and $500 million. And it takes 3 to 5 years to get it running.
Even then, labor costs are a problem. The average wage for a pharmaceutical technician in the U.S. is 4.8 times higher than in China. That means a pill made in Ohio might cost three times as much as the same pill made in Shanghai. Most insurers won’t pay that difference. So companies keep outsourcing.
Some are trying nearshoring-moving production to Mexico or Eastern Europe. One Fortune 500 medical device maker switched from China to Mexico and cut shipping time from 30 days to 7. On-time delivery jumped to 99.2%. But labor costs there are still 15-20% higher than in Asia. And Mexico’s own infrastructure struggles with power outages and water shortages. It’s better, but not perfect.

What’s Working: Diversification and Tech
The smartest companies aren’t choosing between China or the U.S. They’re choosing both-and more. A growing number are using a strategy called multi-shoring: spreading production across three or more countries. One U.S. generic drugmaker now sources its key ingredients from China, India, Germany, and Mexico. If one source fails, the others pick up the slack.
That’s not just theory. In 2025, companies using multi-shoring reported 65% fewer disruption days than those relying on single-source suppliers. One company that dual-sourced its antibiotics saw a 37% drop in costs after two years-and zero shortages. The catch? It takes 18 months to set up and $2.3 million in upfront investment. Small players can’t afford it. That’s why shortages still hit them hardest.
Technology is helping too. AI-powered supply chain tools now predict disruptions before they happen. One manufacturer in Melbourne uses digital twins-virtual replicas of its supply chain-to simulate how a port closure in Singapore would affect insulin shipments to Sydney. They found a backup route through Chile and adjusted orders in advance. No shortage. No panic.
Blockchain is also making headway. Instead of trusting paper certificates from overseas suppliers, companies are now verifying ingredient quality through secure digital ledgers. One Australian hospital group reduced quality disputes by 65% after switching to blockchain verification. It’s not perfect, but it’s a step toward accountability.
The Real Risk: Systemic Collapse
The biggest danger isn’t one factory closing. It’s that multiple failures happen at once. Climate events. Cyberattacks. Trade wars. Geopolitical tensions. All of these are converging. In 2024, a cyberattack on a major Indian API supplier knocked out inventory tracking for 11 days. A heatwave in China shut down power to three manufacturing zones. At the same time, the U.S. imposed new tariffs on pharmaceutical chemicals. The result? A perfect storm of delays.
Small businesses are the most vulnerable. About 90% of global pharmaceutical suppliers are small or mid-sized. They don’t have the cash to diversify. They don’t have the tech to predict risks. And they’re often the first to be cut when big buyers demand lower prices. That’s why the same few suppliers keep getting overworked-and why the system keeps breaking.
Experts warn this isn’t just a supply chain issue. It’s a public health emergency. The World Economic Forum calls it a “systemic shock.” When you tie the life-saving medicines of millions to a handful of factories in unstable regions, you’re gambling with human lives.
What Needs to Change
There’s no magic fix. But here’s what’s working for those who’ve made the shift:
- Diversify suppliers-don’t rely on one country or one company for critical ingredients.
- Invest in inventory buffers-stockpile 15-20% more than you think you need. It’s expensive, but cheaper than a shortage.
- Use digital tools-AI, IoT sensors, and blockchain aren’t luxuries anymore. They’re survival tools.
- Support local production-governments need to offer tax incentives and grants to build domestic API capacity. Australia has started a $400 million fund for this. The U.S. has a similar program under the CHIPS and Science Act.
- Require transparency-hospitals and insurers should demand to know where ingredients come from. If a drug’s supply chain is opaque, it shouldn’t be approved.
Change is slow. But it’s happening. In 2025, 78% of pharmaceutical companies are actively diversifying their supply chains-up from 35% in 2020. That’s progress. But it’s not enough. Until we treat medicine like a public good-not just a commodity-we’ll keep seeing the same shortages, year after year.
What You Can Do
If you’re a patient, ask your pharmacist: “Where is this drug made?” If you’re a healthcare provider, push for suppliers who disclose their sourcing. If you’re a policymaker, fund domestic production. And if you’re a business leader-stop treating medicine like a cost center. It’s a lifeline.
The next time you pick up a prescription, remember: it didn’t just appear on the shelf. It crossed oceans, passed through inspections, survived tariffs, and dodged disruptions. That’s not luck. It’s a system that’s barely holding together. And if we don’t fix it, the next shortage won’t be just a few pills. It could be the one you need to survive.
Why are drug shortages happening now and not before?
Drug shortages are worse now because the global supply chain became too concentrated. In the past, companies had multiple suppliers across different regions. Today, 80% of active ingredients come from just China and India. When one factory shuts down-due to weather, politics, or a cyberattack-the entire system feels it. Add in longer shipping times, new tariffs, and workforce shortages, and the system is far more fragile than it was 10 years ago.
Can we make drugs in Australia or the U.S. instead?
Yes, but it’s expensive. Building a single FDA- or TGA-compliant manufacturing plant for active ingredients costs between $200 million and $500 million and takes 3-5 years. Labor costs are 4-5 times higher than in Asia. That’s why most companies still outsource. But governments are starting to step in-with Australia investing $400 million in local pharma production and the U.S. offering tax credits under the CHIPS Act. It’s slow, but it’s moving.
Are generic drugs more at risk than brand-name drugs?
Yes. Generic drugs have very thin profit margins-sometimes just pennies per pill. Companies don’t invest in backup suppliers or extra inventory because they can’t afford it. Brand-name drugs, like those from Pfizer or Merck, often have higher margins and can afford to stockpile ingredients or use multiple suppliers. That’s why you rarely hear about shortages of brand-name drugs like Humira or Keytruda-but you hear about shortages of metformin or amoxicillin all the time.
How does AI help with drug supply chains?
AI predicts disruptions before they happen. For example, it can analyze weather patterns, port congestion, political unrest, and shipping delays to forecast when a shipment might be late. One Australian drug distributor uses AI to reroute shipments from China to Mexico if a typhoon is coming. It also helps optimize inventory levels so companies don’t overstock or understock. Companies using AI in their supply chains have cut lead times by up to 20% and reduced shortages by 30%.
Is nearshoring to Mexico a good solution?
It’s one of the best options right now. Shipping from Mexico to the U.S. takes 3-5 days instead of 20-30 from China. Labor costs are higher than in Asia, but not as high as in the U.S. Many U.S. and Australian companies are shifting packaging, final assembly, and even some API production to Mexico. One medical device maker cut shipping time from 30 days to 7 and improved on-time delivery to 99.2%. But Mexico has its own risks-power outages, water shortages, and political instability in some regions. It’s not perfect, but it’s better than relying on a single Asian supplier.




Elizabeth Grace December 3, 2025
I filled my amoxicillin prescription last week and the pharmacist said it was from India. I asked if it was safe. He just shrugged. That’s not good enough. My kid’s asthma inhaler? Same story. We’re gambling with lives because someone’s balance sheet looked nice.
Steve Enck December 4, 2025
The structural inefficiencies inherent in the globalization of pharmaceutical production have reached a critical inflection point. The concentration of active pharmaceutical ingredient (API) manufacturing in two geopolitical entities-China and India-constitutes a systemic vulnerability of unprecedented magnitude. The elasticity of supply chains has been sacrificed for marginal cost arbitrage, resulting in a fragile equilibrium that is now demonstrably unsustainable under even minor perturbations. The 15% increase in FDA quality-related recalls is not a statistical anomaly-it is the inevitable consequence of deregulatory capture and profit-maximizing rationality at the expense of public health infrastructure.
Jay Everett December 5, 2025
Y’all are right to be scared-but don’t throw the baby out with the bathwater. 🚨 We’ve got tech that can fix this. AI predicting port delays? Check. Blockchain tracking every gram of API from factory to pharmacy? Check. Companies using multi-shoring (China + India + Germany + Mexico) cut shortages by 65%? DOUBLE CHECK. 🤝 It’s not about going back to 1990s US manufacturing-it’s about building a smarter, distributed network. The money’s there. The will? That’s the only thing we’re missing. Let’s stop treating medicine like a commodity and start treating it like oxygen. 💨
मनोज कुमार December 6, 2025
India making 40 of global generics why blame us when US and EU demand cheap drugs and ignore quality control. Factories follow orders not morals. You want safe drugs pay more or make them yourself
Zed theMartian December 8, 2025
Oh wow, a *surprise* that outsourcing to authoritarian regimes with lax oversight and zero transparency led to a public health crisis? I’m shocked. Shocked, I tell you. Next you’ll tell me that building a nuclear reactor in a floodplain is risky. Maybe if we stopped pretending capitalism is a moral system, we wouldn’t keep designing systems that kill people to save $0.03 per pill.
Ella van Rij December 9, 2025
So… we’re all just supposed to be grateful that our insulin didn’t vanish because a factory in Gujarat had a power outage? And the fact that my doctor had to prescribe me a different generic because the other one was ‘unavailable’ is just… life now? I mean, I guess I should be thankful I’m not dead? 🤷♀️ #PharmaGamble
ATUL BHARDWAJ December 10, 2025
China and India make medicine because they know how to make it. America makes lawsuits and press releases. The cost of making things here is not just money. It is time. It is patience. We do not have that patience anymore
Steve World Shopping December 11, 2025
Let’s be real. This isn’t a supply chain issue-it’s a moral failure. You outsource to countries with no labor rights, no environmental controls, and then act surprised when the system collapses. You didn’t lose control of the supply chain-you gave it away. And now you want a bailout? No. You want accountability. Not tax credits. Not subsidies. Accountability.
Rebecca M. December 12, 2025
I cried when my mom’s chemo got delayed. Not because I’m dramatic. Because it was *supposed* to be safe. And now I’m supposed to be grateful that my pharmacy can’t even tell me where the damn pill came from? This isn’t capitalism. This is a horror movie where the villain is a spreadsheet.
Alicia Marks December 13, 2025
Small business owners: you’re not alone. I run a clinic. We’ve been hit hard. But here’s what’s working-joining a regional buying co-op that diversifies suppliers. It’s not perfect, but we haven’t had a shortage in 8 months. And yes, it costs more. But our patients are alive. That’s the ROI.
Shannara Jenkins December 14, 2025
Just wanted to say thank you for writing this. I’ve been a nurse for 18 years and I’ve watched this happen slowly. No one talks about the nurses who have to call families and say, ‘We don’t have the med today.’ That silence? It’s heavy. But change is coming. I’ve seen clinics start asking suppliers for origin certificates. It’s small, but it’s something. Keep pushing. We’re with you.
dave nevogt December 15, 2025
There’s a deeper question here that no one’s asking: what does it mean to live in a society where the tools of survival-medication-are treated as disposable commodities? We’ve outsourced not just production, but responsibility. We no longer feel the weight of the system because we don’t see the people who make it. We don’t know the workers in the factories in Zhejiang or Gujarat. We don’t know the pharmacists who ration pills. We don’t know the children who miss doses. And so we become numb. The crisis isn’t just in the supply chain-it’s in our collective moral imagination. To fix the medicine, we must first relearn how to care.