International Supply Chains: How Global Drug Networks Keep Medicines Available
When you pick up a bottle of generic lisinopril or a box of insulin, you’re holding a product that may have been manufactured in India, packaged in Germany, shipped through Singapore, and cleared by U.S. customs—all before reaching your local pharmacy. This is the international supply chain, the global network of manufacturers, distributors, regulators, and logistics providers that moves pharmaceuticals across borders. Also known as the pharmaceutical supply chain, it’s not just about moving boxes—it’s about keeping people alive when they need medication today, not next month.
Behind every pill is a chain of decisions: Who makes it? Where are the raw ingredients sourced? Is the factory inspected by the FDA or EMA? How many warehouses hold backup stock? The generic drug distribution, the system that delivers low-cost versions of brand-name drugs to millions, runs on razor-thin profits and zero room for error. A single factory shutdown in China or a port strike in Los Angeles can trigger a nationwide shortage of antibiotics or blood pressure meds. That’s why the drug safety, the ongoing process of monitoring how medicines are made, shipped, and used across countries isn’t just about side effects—it’s about making sure the right drug gets to the right person, on time, every time.
Most people think of drug shortages as rare accidents. But they’re actually the result of predictable failures: one supplier dominating a market, lack of backup manufacturers, or cost-cutting that ignores inventory buffers. The international supply chains that deliver generic drugs are efficient—but they’re also fragile. When a company in India stops making a key active ingredient, or when shipping costs spike because of fuel prices or tariffs, pharmacies don’t just raise prices—they run out. That’s why posts here cover everything from how the FDA tracks drug safety through MedWatch, to why the Orange Book matters for generic substitutions, to how supply chain economics shape whether your thyroid med is in stock this week.
You won’t find flashy headlines here about global trade deals or political debates. What you will find are real stories about how logistics, regulation, and manufacturing decisions impact your health. Whether it’s understanding why insulin biosimilars are still hard to get, how Black Box Warnings tie into factory audits, or why fiber supplements can interfere with meds shipped across oceans—this collection connects the dots between the global system and your medicine cabinet. The next time you pick up a prescription, remember: someone somewhere in the world just made sure it was safe, legal, and ready for you. This is how that happened.
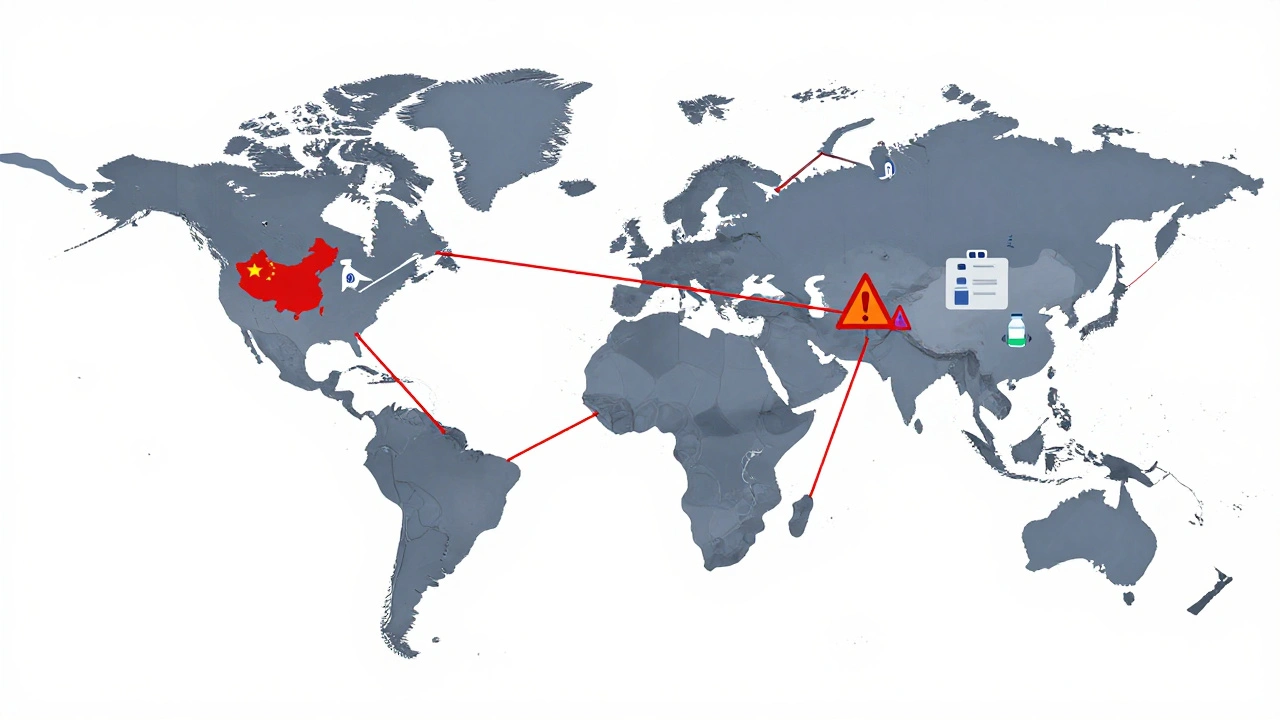
International Supply Chains: Why Foreign Manufacturing Is Causing Drug Shortages in 2025
International supply chains for pharmaceuticals are under strain, causing widespread drug shortages in 2025. Over 80% of active ingredients come from China and India, making the system vulnerable to disruptions. Here’s how it’s happening-and what’s being done to fix it.