Adverse Event Reporting: What It Is and Why It Saves Lives
When a medication causes an unexpected or harmful reaction, that’s called an adverse event reporting, the systematic process of documenting harmful side effects from drugs, vaccines, or medical devices to protect public health. Also known as pharmacovigilance, it’s not just paperwork—it’s the early warning system that keeps dangerous drugs off shelves and alerts doctors to hidden risks. Every time someone reports a bad reaction—whether it’s a rash after taking a new antibiotic or liver damage from a long-term painkiller—you’re helping build a safer medicine system.
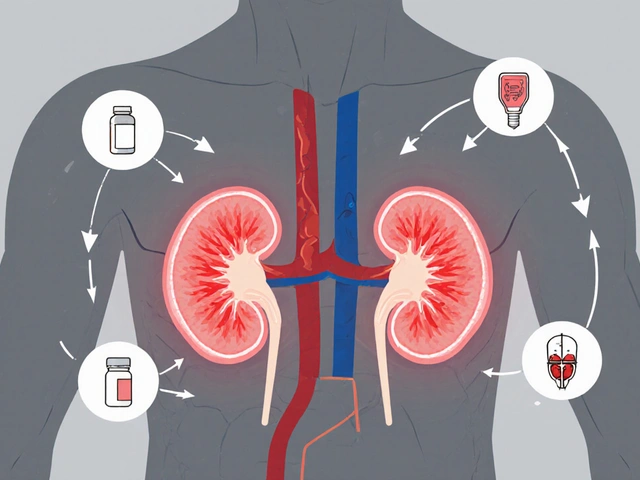
These reports feed into databases run by the FDA, the U.S. agency responsible for approving and monitoring drugs for safety, and global networks like the WHO’s Uppsala Monitoring Centre. But it’s not just regulators who use this data. Pharmacists use it to spot dangerous drug interactions, when two or more medications combine to cause unexpected harm. Doctors use it to choose safer options for patients with complex conditions. And patients? They use it to know what signs to watch for. One report might seem small, but when thousands pile up, they reveal patterns no clinical trial ever caught—like how a popular painkiller suddenly caused rare kidney failures in older adults, or how a new antidepressant triggered suicidal thoughts in teens.
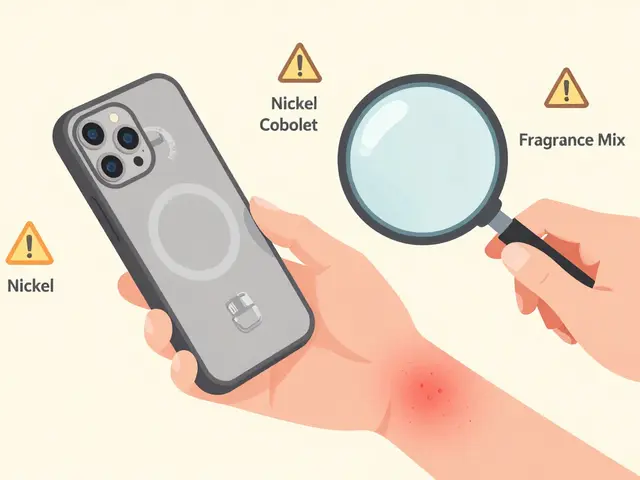
Most reports come from healthcare pros, but patients and caregivers are just as important. If you notice unusual symptoms after starting a new drug—like unexplained bruising, sudden confusion, or trouble breathing—don’t wait. Talk to your pharmacist, write it down, and file a report. You don’t need to prove it was the drug. Just describe what happened, when, and what you were taking. The system is built to catch the unexpected. And it’s working. Black box warnings, drug recalls, and safety updates? They often start with a single report from someone who noticed something didn’t feel right.
Below, you’ll find real-world guides on spotting dangerous side effects, understanding FDA safety alerts, managing opioid reactions, and avoiding hidden risks with common meds. These aren’t theoretical—they’re based on actual reports that changed how drugs are used. Your next report could be the one that saves someone’s life.
MedWatch System Explained: How FDA Tracks Drug and Device Safety
MedWatch is the FDA's system for collecting safety reports on drugs, devices, and other medical products. Learn how it works, who reports, and why your report matters for public health.