Generic Drugs: What They Are, How They Work, and Why They Matter
When you hear generic drugs, medications that contain the same active ingredients as brand-name drugs but are sold under their chemical name. Also known as non-brand medications, they are the backbone of affordable healthcare in the U.S. and around the world. They aren’t cheaper because they’re weaker—they’re cheaper because their manufacturers didn’t pay millions to develop the drug from scratch. The FDA requires them to work the same way, in the same amount, and with the same safety profile as the original. If your doctor prescribes Lipitor, and you pick up atorvastatin instead, you’re getting the exact same molecule. The difference is in the price, not the power.
Many people worry that generic drugs are somehow inferior. That’s not true. A 2019 study from the FDA analyzed over 1,000 generic drugs and found no meaningful difference in effectiveness compared to their brand-name counterparts. Even complex drugs like insulin biosimilars—like the ones discussed in our posts on Lantus alternatives—are held to the same strict standards. What changes are the inactive ingredients: fillers, dyes, or coatings. These don’t affect how the drug works in your body, but they might cause rare allergies or make a pill harder to swallow. That’s why some people feel different switching brands—but it’s rarely because the active drug changed.
Not all generics are created equal in the eyes of doctors. Some hesitate to switch patients from brand to generic for conditions like epilepsy or thyroid disease, where tiny differences in absorption could matter. But that’s not because generics are unreliable—it’s because those conditions need rock-solid consistency. For most people, though, switching to a generic is safe, smart, and saves hundreds a year. If you’re on a long-term medication, ask your pharmacist: Is there a generic? If you’re paying out of pocket, it’s often the only sensible choice.
Related to this are biosimilars, biologic drugs that are highly similar to branded biologics but not exact copies, often used for conditions like diabetes, arthritis, and cancer. These aren’t traditional generics—they’re more complex, made from living cells, and cost 30% less than the original. They’re part of the same story: making powerful medicines affordable. And then there’s the idea of brand name drugs, medications originally developed and marketed by pharmaceutical companies under a patent-protected name. These are the ones with the flashy ads and the high price tags. But once the patent expires, generics enter the market—and prices drop fast.
What you’ll find below is a collection of real-world stories about how these drugs interact with your body, your wallet, and your health. From insulin biosimilars to antibiotic alternatives, from fiber supplements that block absorption to opioid side effects that generics don’t fix—each post cuts through the noise. You’ll learn when generics are the obvious choice, when you should double-check, and when a brand might still be worth the cost. No fluff. No marketing. Just what you need to know to make smarter decisions about your meds.
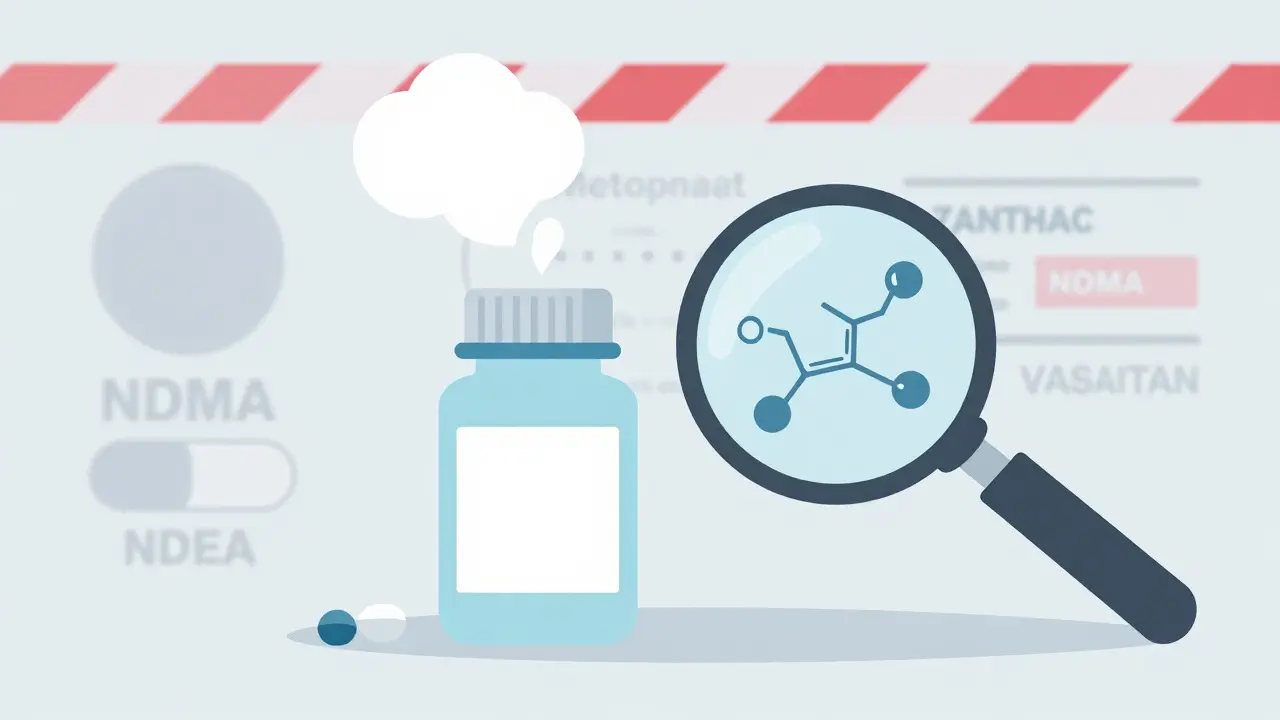
Nitrosamine Contamination in Generic Drugs: Recent Recalls and Regulatory Shifts
Nitrosamine contamination in generic drugs has led to over 500 FDA recalls since 2018. Learn which medications were affected, how regulators responded, and what this means for patient safety.
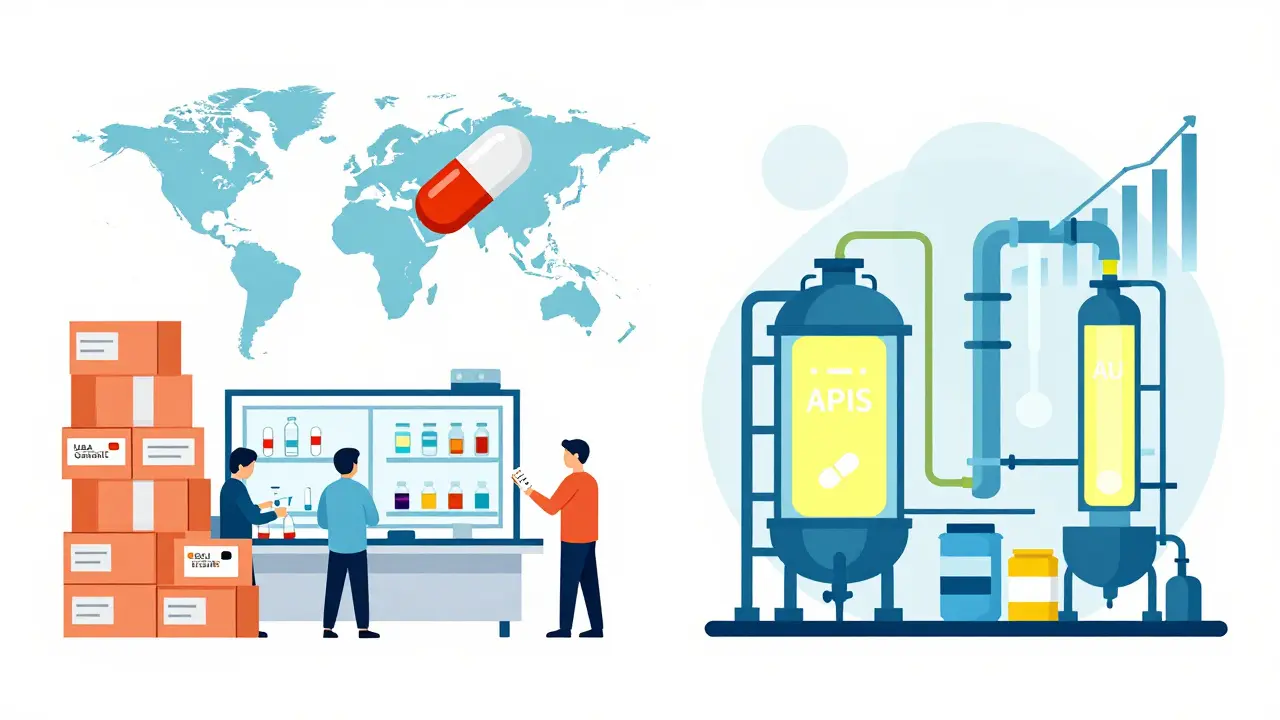
Asian Generic Markets: How India and China Dominate Global Pharma Supply Chains
India and China dominate the global generic drug market, with India producing affordable pills and China supplying the essential chemicals behind them. Emerging economies like Vietnam and Cambodia are now carving out their own niches, reshaping global pharma supply chains.
Complex Generic Formulations: Why Proving Bioequivalence Is So Hard
Proving bioequivalence for complex generics like inhalers, creams, and injectables is far harder than for simple pills. Learn why standard tests fail, how reverse-engineering works, and what’s being done to bring affordable versions to market.
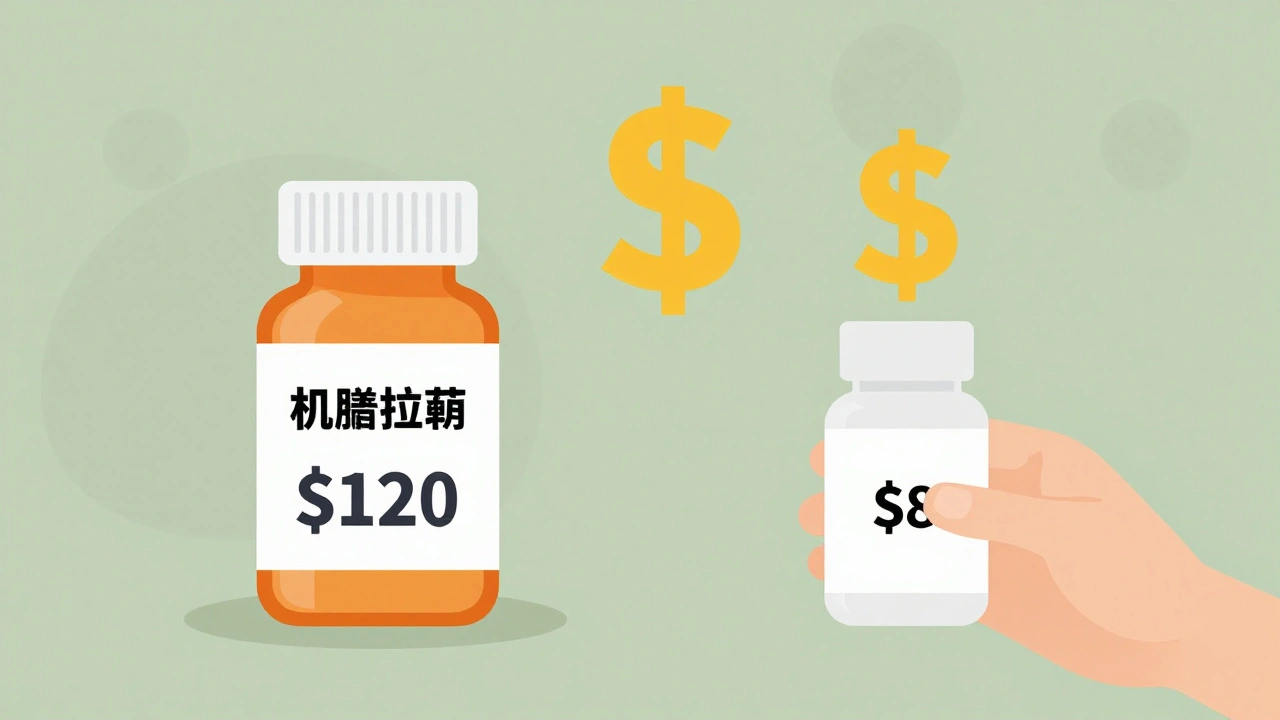
Out-of-Pocket Costs: How Generics Slash Your Medication Bills
Generics cut out-of-pocket drug costs by up to 90%. Learn how brand-name prices compare, why you're still overpaying, and how to save hundreds yearly with simple steps - no insurance tricks needed.
Generic vs. Brand Name Drugs: What You Need to Know About Bioequivalence and Cost Savings
Generic drugs are just as effective as brand-name versions, saving patients up to 85% on medication costs. Learn how bioequivalence testing ensures safety and when to watch for subtle differences.
FDA Orange Book: How Approved Generic Drugs Are Listed
The FDA Orange Book lists approved generic drugs and shows which ones are therapeutically equivalent to brand-name drugs. It's the official guide for pharmacists, insurers, and patients to understand drug substitution rules.