Pharmaceutical Supply Chain: How Drugs Get from Factory to Your Pharmacy
When you pick up a prescription, you’re holding the end result of a complex pharmaceutical supply chain, the end-to-end system that moves medications from raw ingredients to patients, involving manufacturers, distributors, regulators, and pharmacies. Also known as the drug distribution network, it’s not just about shipping boxes—it’s about ensuring every pill, injection, or inhaler meets strict safety and quality standards before it reaches you.
This system relies on tightly controlled steps: raw materials are sourced, formulated into final products, tested for purity, labeled correctly, and shipped under specific conditions. Along the way, agencies like the FDA, the U.S. agency that approves drugs, monitors safety, and enforces labeling rules step in to verify that what’s in the bottle matches what’s on the label. The FDA Orange Book, the official list of approved generic drugs and their therapeutic equivalents to brand-name drugs, is one tool that helps pharmacists and insurers know which substitutions are safe. But even with these checks, risks exist—like counterfeit drugs slipping through, temperature-sensitive meds being stored wrong, or delays causing shortages. That’s why knowing how the chain works helps you ask better questions when something seems off.
The supply chain isn’t just about big companies and regulations—it affects your daily health. If a generic version of your medication isn’t properly validated, it might not work the same. If a drug’s transport temperature isn’t controlled, its potency drops. If a black box warning is buried in paperwork, you might not know the risks. That’s why posts here break down real issues: how to read FDA interaction tables, why black box warnings matter, how fiber supplements interfere with absorption, and how insulin biosimilars are changing cost and access. These aren’t abstract topics—they’re direct outcomes of how the supply chain functions, or fails, at different points.
What you’ll find below are clear, practical guides written by people who’ve seen the gaps in this system. From how fentanyl patches are monitored to why certain opioid side effects are unavoidable, each article ties back to one truth: your safety depends on what happens before you even see the bottle. This isn’t just about drugs—it’s about trust, transparency, and knowing who’s responsible when things go wrong.
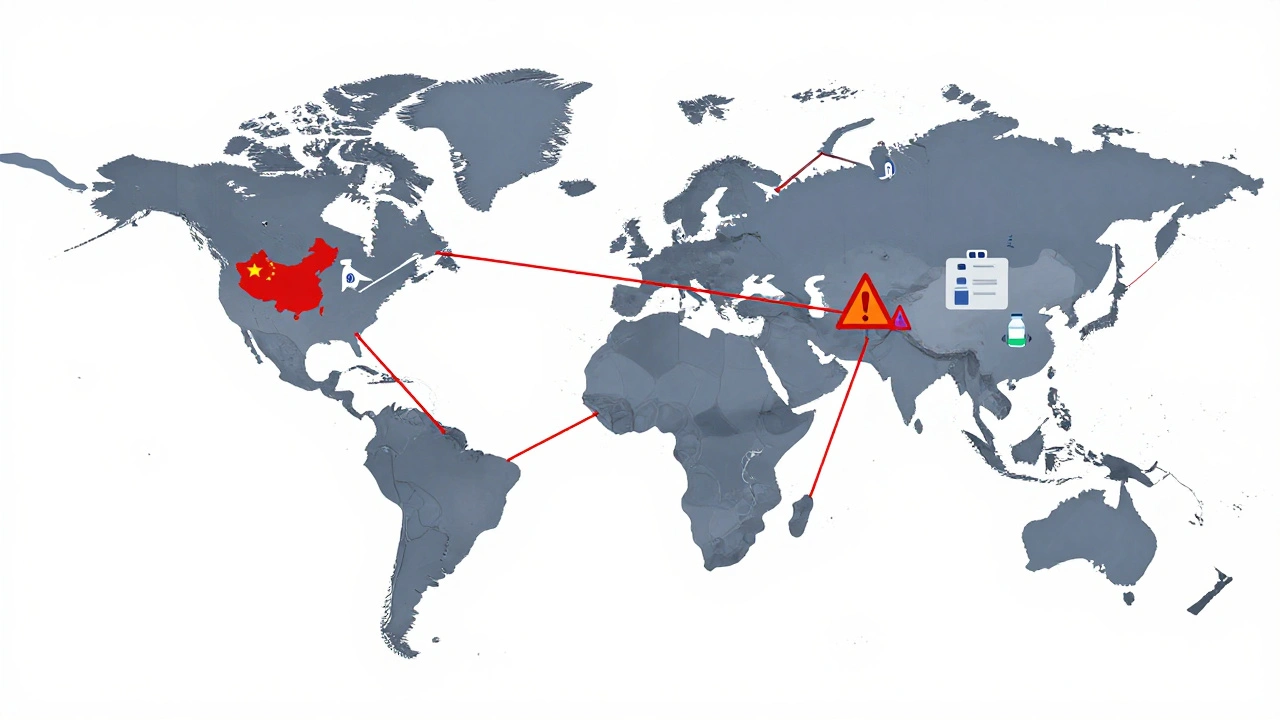
International Supply Chains: Why Foreign Manufacturing Is Causing Drug Shortages in 2025
International supply chains for pharmaceuticals are under strain, causing widespread drug shortages in 2025. Over 80% of active ingredients come from China and India, making the system vulnerable to disruptions. Here’s how it’s happening-and what’s being done to fix it.
Supply Chain Economics: How Efficiency Drives Generic Drug Distribution
Generic drug distribution survives on razor-thin margins, but efficiency isn't just about cost-cutting-it's about preventing life-threatening shortages. Learn how data, technology, and smarter inventory are reshaping the system.